- Volume 64 , Number 3
- Page: 333–5
Effect of Mycobacterium leprae on peripheral nerve protein phosphorylation; a preliminary study
To the Editor:
Protein phosphorylation plays an important role in the regulation of cellular metabolism (1). An important question that needs to be answered in leprosy is how Mycobacterium leprae interacts with the host cell signal transduction pathways (5). We report here the in vitro effect of M. leprae on rat and human peripheral nerve phosphorylation.
Tris hydroxymethylaminomethane (Tris), phenyl methyl sulfonyl fluoride, benzamidine hydrochloride, Triton X-100, beta mercaptoethanol and trypsin were obtained from Sigma Chemical Company, St. Louis, Missouri, U.S.A. Other reagents used in the study were of analytical grade. Gamma 32P ATP was obtained from Bhabha Atomic Research Centre, Bombay, India. Normal human peripheral nerves were obtained from amputated limbs, the conditions being osteogenic carcinoma or soft tissue sarcomas. Tibial nerves were traced and collected immediately after surgery, and they were immediately frozen at -20ºC until their use (usually within a month). Rats were sacrificed by cervical dislocation and the peripheral nerves (sciatic, tibial and sural) were dissected and processed immediately at 4ºC. The human and rat nerve samples were cleared of the connective tissue and minced. The minced tissue was homogenized in a glass teflon homogenizer in 10 volumes of Tris-HCl buffer, pH 7.6 (0.02 M) containing phenyl methyl sulfonyl fluoride (0.002 M), benzamidine hydrochloride (1 mg/ml) and 0.1% (v/v) Triton X-100 (10 ml/g wet tissue). The homogenate was centrifuged at 1000 x g for 20 min. The supernatant was used for the study of protein phosphorylation. Protein was estimated according to Lowry, el al. (4).
M. leprae isolation. M. leprae were isolated from skin biopsies of leprosy patients and mouse foot pads infected with M, leprae. The tissues were minced with scissors and homogenized in a glass homogenizer in sterile 0.05 M phosphate buffer (pH 7.0). After coarse tissue debris was removed by low speed centrifugation (100 x g for 3 min), sterile 0.5% w/v trypsin solution was added to the bacillary suspension at a final concentration of 0.05%. This suspension was incubated at 37ºC for 45 min and then ccntrifuged (1500 x g for 15 min). The sediment was suspended in 0.05 M phosphate buffer (pH 7.0) and treated with NaOH at a final concentration of 0.25 N for 10 min at 37ºC. The bacilli suspension was then neutralized with 0.1 N HC1 and centrifuged. The pellet bacilli were suspended in 0.01 M Tris-HCl buffer (pH 7.2) containing 0.15 M NaCl, 0.001 M MgCl2, and 0.1 % v/v Triton X-100. The suspension was again ccntrifuged and the bacillary pellet was finally suspended in the buffer used for nerve tissue homogenization. After purification the bacilli were acid fast and r;-diphenol oxidase positive (World Health Organization. Laboratory techniques for leprosy and Appendix 2. Chapter 7. Identification of M. leprae. Geneva: World Health Organization, 1987, pp. 107-136).
Protein phosphorylation. Unless otherwise indicated, the reaction mixture for protein phosphorylation consisted of 100 µ g protein of the nerve homogenate 1000 x g supernatant, 0.02 M Tris-HCl buffer, pH 7.5, 0.02 M MgCL and 1.3 nM gamma 32P ATP (2 x 106 DPM) in a total volume of 150 µ l. The reaction was initiated by the addition of 32P ATP and incubation was carried out at 37ºC for 5 min. The reaction was terminated by the addition of sodium dodecyl sulfate (SDS)-dissociation buffer, heated at 100ºC for 3 min and subjected to SDS-gel electrophoresis on 10% or 12.5% gels according to Laemmli (3). The stained gel was destained, dried on a gel dryer, and kept in contact with X-ray film at -70ºC for 7 to 10 days for autoradiography (6). Molecular weight markers used were myosin (205 kDa), beta-galactosidase (116 kDa), phosphorylase B (97 kDa), bovine serum albumin (66 kDa), egg albumin (45 kDa) and carbonic anhydrase (29 kDa).
To investigate the effect of M. leprae on peripheral nerve phosphorylation, 50 µ l containing 105 bacilli were added to the reaction mixture prior to the initiation of the reaction by gamma 32P-labeled ATP.
Autoradiograms of the reaction mixture in which rat peripheral nerve extract was incubated with gamma 32P ATP under normal assay conditions showed various phosphorylated bands (Fig. 1, lane 2). The major phosphorylated band was at 28-30 kDa. When M. leprae were added to the phosphorylation mixture, there was a significant decrease in phosphorylation of 28-30 kDa (Fig. 1, lane 1 ). M. leprae alone in the reaction mixture showed no phosphorylated bands (Fig. 1, lane 3). Essentially similar results were obtained for human peripheral nerve. A protein band at 25 kDa that was phosphorylated in the human peripheral nerve (Fig. 2, lane 1) was significantly decreased in the presence of added M. leprae (Fig. 2, lane 3).
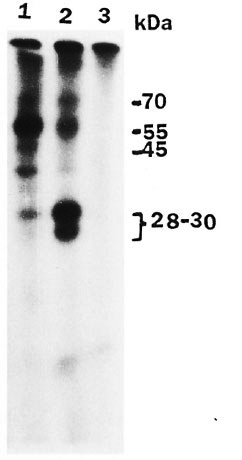
Fig. 1. Autoradiogram showing effect of M. leprae on phosphorylation of rat peripheral nerve pro-teins. Assay was carried out as described in text and SDS-gel electrophoresis was done on 12.5% gel. Lane1 = reaction mixture for phosphorylation with addedM. leprae; lane 2 = reaction mixture; lane 3 = M. leprae alone without peripheral nerve extract in reaction mixture.
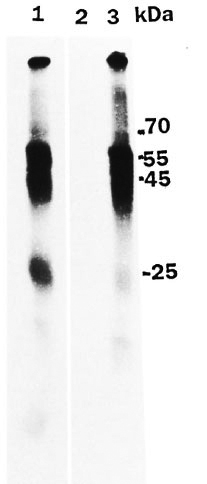
Fig. 2. Autoradiogram showing effect of M. leprae on human peripheral nerve protein phosphoryla-tion. Assay was carried out as described in text andSDS-gel electrophoresis was done on 10% gel. Lane I= reaction mixture for phosphorylation; lane 2 = M. leprae alone without peripheral nerve extract in reac-tion mixture; lane 3 = reaction mixture with added M. leprae .
Phosphorylation using peripheral nerves from rats of different ages (from 1 to 30 weeks) showed the presence of the 32P-phosphorylated 28-30 kDa protein, the maximal phosphorylation being in rats at age 4 weeks and above (Fig. 3, lanes 1 to 5). with added M. leprae, phosphorylation of the 28-30 kDa protein was significantly reduced in all of the age groups at or above 4 weeks (Fig. 3, lanes 6 to 8).
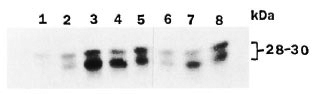
Fig. 3. Autoradiogram showing phosphorylationof 28-30 kDa protein hand following incubation ofgamma 32P ATP with rat peripheral nerve (differentages) 1000 x g supernatant. Assay and SDS-gel electrophoresis on 12.5% gel were conducted as describedin text. Lanes 1 to 5 had reaction mixture for phos-phorylation and lanes 6 to 8 had added M. leprae to the reaction mixture. The age of rat used was 1 week (lane1), 2 weeks (lane 2), 4 weeks (lanes 3 and 6), 10 weeks(lanes 4 and 7) and 30 weeks (lanes 5 and 8). An equa lamount of protein (100 µg) was used in all reaction mixtures.
Phosphorylation of rat peripheral nerve 28-30 kDa protein has been well characterized (2,7,8). Studies in our laboratory have identified the 25-kDa protein of human peripheral nerve and the 28-30 kDa protein of rat peripheral nerve to be glycoproteins which could be phosphorylated (unpublished data). The molecular weights of these proteins appeared similar to the Po protein(2,7). In the present study, the addition of M. leprae caused a decrease in phosphorylation of the 28-30-kDa and 25-kDa proteins in rat and human peripheral nerves, respectively. This could be due to any of the following reasons: a) M. leprae alters the protein kinases; b) M. leprae alters protein phosphatase; c) M. leprae could interact or bind to specific nerve proteins blocking substrate sites. This in vitro observation might have possible implications in M. lep rae-host interactions. The significance of this finding is currently being investigated.
- Lavanya M. Suneetha, M.Sc, Ph.D.
Research Associate
Neurochemistry Laboratory
Christian Medical College Hospital
Vellore 632 004
N.A.A. District
Tamil Nadu, India
- Charles K. Job, M.D., F.R.C.Path.
Consultant Pathologist
St. Thomas Hospital and Leprosy Centre
Chettupattu 606 801
T.S. District
Tamil Nadu, India
- Aiylam S. Balasubramanian, M.Sc, Ph.D.
Professor of Biochemistry
Neurochemistry Laboratory
Christian Medical College Hospital
Vellore 632 004
N.A.A. District
Tamil Nadu, India
Acknowledgment. We acknowledge with gratitude the research associateship offered to Dr. Lavanya M. Suneetha by the Council of Scientific and Industrial Research, New Delhi. We are thankful to Mr. Pugazenthi, St. Thomas Hospital, Chettupattu, for technical help and to Dr. Sujai K. Suneetha of the Schieffelin Leprosy Research Centre, Karigiri, for helpful discussions.
REFERENCES
1. Edelman, A. M., Blumenthal, D. K. and Krebs, E. G. Protein serine/threonine kinases. Ann. Rev. Biochem. 56(1987)567-613.
2. Hilmi, S., Fournier, M., Valeins, II., Gander, J. and Bennet, J. Myelin Po glycoprotein: identification of the phosphorylated site phosphorylated in vitro and in vivo by endogenous protein kinases. J. Neurochem. 64(1995)902-907.
3. Laemmli, U. K. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nature 227(1970)680-685.
4. Lowry, O. H., Rosenbrough, N. J., Farr, A. L. and Randall, R. J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 193(1951)265-275.
5. Ottenhoff, T. H. M. Immunology of leprosy; new developments. Trop. Geogr. Med. 46(1994)72-80.
6. Ramamoorthy, S. and Balasuhramanian, A. S. A Zn2+ dependent tyrosine phosphorylation of a 68 kDa protein and its differentiation from Mg2+ dependent tyrosine phosphorylation in sheep platelets. Arch. Biochem. Biophys. 286(1991)433-440.
7. Suzuki, M., Sakamato, Y, Kitamura, K., Fukunaga, K., Yamamato, H., Miyamoto, E. and Uyemura, K. Phosphorylation of Po glycoprotein in peripheral nerve myelin. J. Neurochem. 55(1990)1966-1971.
8. Wiggins, R. C. and Morell, P. Phosphorylation and fucosylation of myelin proteins in vitro in sciatic nerve from developing rats. J. Neurochem. 34(1980)627-634.
Reprint requests to Dr. Balasubramanian.