- Volume 64 , Number 3
- Page: 336–9
Electron-microscopic study of negative Mitsuda reaction in nine-banded armadillos and lessons to be learned
To the Editor:
A negative Mitsuda reaction in a leprosy patient indicates the absence of a cell-mediated immune response to Mycobacterium leprae and, therefore, either the presence of generalized lepromatous disease or a tendency to progress toward it. In a normal person it indicates the inability to destroy M. leprae and, therefore, he/she is liable to become infected if exposed and, when infected, more likely to develop lepromatous leprosy (3). It is not yet clear whether this anergy to M. leprae is inherent or acquired (2). There have been several histopathological studies of the Mitsuda reaction (4,6,8,11), but an electron-microscope study, especially of the negative reaction, is not found in the literature. In this study we describe the histopathologic and electron-microscopic appearance of the negative Mitsuda reaction in nine-banded armadillos and discuss its significance.
Three known lepromin-negative armadillos were chosen. An armadillo-derived, autoclaved, saline suspension of 1.7 x 108ml M. leprae was prepared and 0.1 ml of the suspension was intradermally injected into the abdominal skin of the armadillos. The injected site was circled with a skin-marking pencil. It is well known that at 21 days the inflammatory cells present at a negative lepromin site are very few, widely dispersed in the dermis, and are occasionally difficult to find. Therefore, it was decided to biopsy the lepromin site on the 10th day using a 5-mm punch.
The biopsy tissues obtained were bisected; one half was fixed in 10% buffered, neutral formalin and processed for paraffin sections (5 µ m sections were made); one section was stained with hematoxylin and eosin (H&E) and the other with a modified File's technique for acid-fast bacilli (AFB)(5). The other half of the tissue was cut into 1-mm cubes and fixed in 5% glutaraldehyde at 4ºC for 4 hr, washed with sodium cacodylate buffer at pH 7.3, postfixed in 1% osmium tetroxide, processed for embedding in Spurrs resin (Polysciences, Inc., Warrington, Pennsylvania, U.S.A.), and ultra-thin sections were made for examination under a Philips EM.410 electron microscope.
Histopathologic examination showed no significant change in the epidermis. In the dermis there were several scattered spindle-shaped, fibroblast-like macrophages with their long thin processes between collagen bundles distributed in a large area of the section. Also present were small focal collections of macrophages having a pink granular cytoplasm (Fig. 1). There were no recognizable plasma cells. Lymphocytes were very scanty. Neutrophils were absent. The acid-fast stain showed granular bacilli in clumps inside macrophages. Many organisms were lying free in tissue spaces and a few were present in endothelial cells of capillaries.
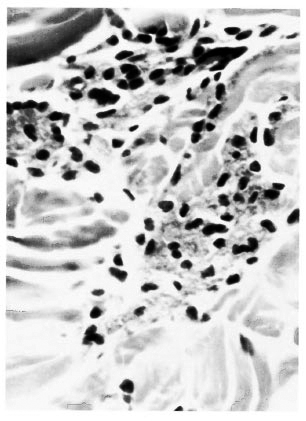
Fig. 1 Photomicrograph showing small focal collections of macrophages with abundant granular cytoplasm in the dermis (H&E x400).
An electron-microscopic study yielded very interesting and informative findings. Several thin, elongated macrophages with long processes in between collagen bundles containing clumps of M. leprae were seen (Fig. 2). There were also many macrophages around and in close apposition to several small lymph and blood vessels (Fig. 3). All of them had many intracellular M. leprae with an accumulation of electron-transparent material around them. Macrophages containing M. leprae were found inside a few dilated lymph channels and occasional blood vessels (Figs. 4 and 5). The endothelial cells lining the capillaries and lymph vessels also showed M. leprae (Fig. 5). Many fragmented M. leprae were seen lying free in the interstitial spaces of the dermis. No plasma cells were identified, and lymphocytes were very rare.
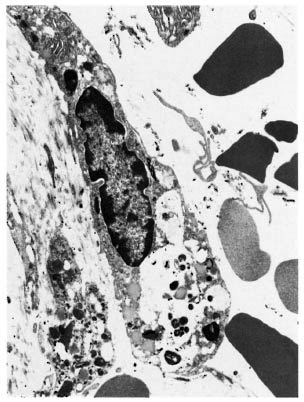
Fig. 2. Electronmicrograph of a spindle-shaped macrophage lying between collagen bundles containing many M. leprae (x7000).
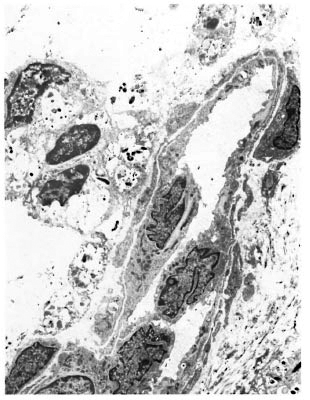
Fig. 3. Electronmicrograph of a blood vessel surrounded by macrophages containing M. leprae (x7000).
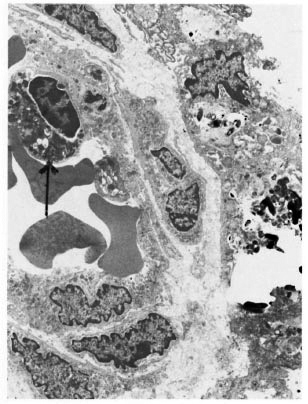
Fig. 4. Electronmicrograph of a blood vessel showing in its lumen a macrophage (↑) with several M. leprae (x7000).
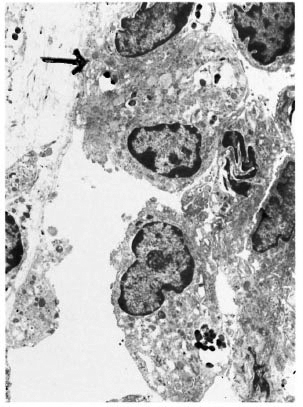
Fig. 5. Electronmicrograph of a lymphatic vessel with macrophages in its lumen. An endothelial cell (→) and the macrophages contain many M. leprae (x7000).
When 1.6 x 107 M. leprae were injected intradermally into a lepromin-negative subject only a minimal inflammatory reaction was initiated which on the l0th day consisted almost entirely of a small number of macrophages, some scattered and others in small focal collections in the dermis. The blood monocytes that came into the site ingested as much of the bacilli as possible, migrated back into the lymph and blood vessels, and were carried away to regional lymph nodes and other remote organs. It was observed that the macrophages had not phagocytized all of the organisms injected because a sizable amount of bacilli were still seen lying free in interstitial spaces. In a study evaluating the efficacy of a vaccine, the sites which gave an earlier negative Mitsuda response turned positive many months later following the administration of an effective vaccine, thus showing the persistence of M. leprae antigen at the injected site for many months (1).
It is quite possible that in lepromin-negative armadillos the macrophages have a genetic defect similar to that discovered in murine macrophages (10,12) and, therefore, they were unable to secrete T-cell-amplifying monokines such as interleukin-1 and tumor necrosis factor-alpha. Defective macrophage function in lepromatous disease has been pointed out by many other workers (7,9). Hardly any lymphocytes were found at the injected site. It is obvious that there were no cytokines either to stimulate the recruitment of inflammatory cells or to immobilize them at the site and to aid in the formation of a protective granuloma.
The fact that some portion of the killed bacilli were left behind lying free in the tissue even on the l0th day without being handled by phagocytes, gives credence to the thesis that if M. leprae get in, in a susceptible host they will be exposed to and enter parenchymal cells like endothelial cells, as shown in this study, and also in Schwann cells and smooth muscle cells even at the early stage of infection. If the infected person develops immunity, he may develop a tuberculoid lesion at the site of entry where the organisms persist in interstitial tissue and parenchyma cells. Failure to develop immunity will result in lepromatous disease.
Study of the cytokine kinetics of the negative lepromin reaction in normal human subjects will yield valuable results to clarify the immunologic defect in lepromatous leprosy.
- Charles K. Job, M.D., F.R.C. Path.
Consultant Pathologist
St. Thomas Hospital and Leprosy Centre
Chettupattu 606 801
T. S. District
Tamil Nadu, India
- Gregory T. McCormick, B.S.
Laboratory Technician
- Richard W. Truman, Ph.D.
Chief
Microbiology Research Department
- Angelina T. Deming, B.S.
Laboratory Technician
- Maggie T. Landry, B.S.
Laboratory Technician
- Roena M. Hunt, B.S.
Laboratory Technician
Laboratory Research Branch
GWL Hansen's Disease Center at Louisiana State University
P.O. Box 25072
Baton Rouge, LA 70894, U.S.A.
Acknowledgment. Our grateful thanks are due to Dr. David Scollard, Chief of Pathology, GWL HD Center, for permission to use the electron-microscope: to Dr. J. L. Krahenbuhl, Chief of the Laboratory Research Branch, GWL HD Center; to Dr. W. Felton Ross, American Leprosy Missions, Inc., for financing part of Dr. Job's visit, and to Miss K. Jayanthi for secretarial help.
REFERENCES
1. Convit, J., Ulrich, M. and Aranzazu, N. Vaccination in leprosy - observations and interpretations. (Editorial) Int. J. Lepr. 48(1980)62-65.
2. Dharmendra. Leprosy, Vol.2. Bombay: Samant and Co., 1985, pp. 999-1061.
3. Dharmendra and Chatterjee, K. R. Prognostic value of the lepromin test in contacts of leprosy cases. Lepr. India 27(1955)149-152.
4. Desikan, K. V. and Girdar, B. K. Pathology of late lepromin reaction in cases of leprosy across the spectrum. Indian J. Lepr. 66(1984)112-113.
5. Job, C. K. and Chacko, C. J. G. A modification of Fite's stain for demonstration of M. leprae in tissue sections. Indian J. Lepr. 58(1986)17-18.
6. Job, C. K., Kirchheimer, W. F. and Sanchez, R. M. Tissue response to lepromin an index of susceptibility of armadillo to M. leprae infection-a preliminary report. Int. J. Lepr. 50(182)177-182.
7. Marolia, J.. Robinson, P. and Mahadevan, P. R. A complex component modulating immune deficient cells in leprosy patients leading to loss of viability of M. leprae- a possible vaccine. Clin. Exp. Immunol. 79(1990)7-14.
8. Mistry, N. F, Birdi, T. J., Uplekar, M. and Antia, N. H. A sequential histopathological study of lepromin reaction in leprosy patients. IRCS Med. Sci. 12(1984)287-288.
9. Nathan, C. F, Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant interferon in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
10. Schurr, F, Morgan, K., Gros, P. and Skamene, E. Genetics of leprosy. Am. J. Trop. Med. Hyg. 44(1991)4-11.
11. Thomas, J., Joseph, M., Ramanujam, K., Chacko, C. J. G. and Job, C. K. Histology of the Mitsuda reaction and its significance. Lepr. Rev. 51(1980)329-340.
12. Vidal, S. M., Malo, D., Vogan, K., Skamene, E. and Gros, P. Natural resistance to infection with intracellular parasites: isolation of a candidate for BCG. Cell 73(1993)469-485.