- Volume 64 , Number 4
- Page: 383–91
Effect of HIV infection on leprosy: a three-year survey in Bamako, Mali
ABSTRACT
F rom February 1992 until June 1994, all patients with histologically proven leprosy examined at the Leprology Unit of the Institut Marchoux in Bamako, Mali, were screened for HIV serology. In total, 740 leprosy patients have been tested; 553 known, previously treated leprosy cases and 187 new cases, mainly self-reporting and referred cases. The global seroprevalence in the sample was 1.5% ( 11/740), and increased f rom 1.3% in 1992 to 3.1% in 1994. HIV seroprevalence was higher in paucibacillary (PB) than in multibacillary (MB) cases (3.8% versus 0.8%, p < 0.05), and was slightly higher in new cases than in known, already treated cases (2.1 % versus 1.3%), although not significantly. Among the 553 known, already treated leprosy patients, 1 out of 7 HIV-seropositive patients relapsed, as opposed to 34 out of 546 HIV seronegative cases ( 14.2% versus 6.2%, p = 0.36). Among the new cases, none of the 37 patients with reaction and/or neuritis was HIV positive. In known, treated leprosy cases, there was no difference in the frequency of reactions and/or neuritis between HIV-positive and HIV-negative cases. Migration in a neighboring country appeared to be a risk factor for HIV seropositivity in our sample ( χ 2 = 4.5, p = 0.04). In order to estimate the association of HIV with leprosy as compared to the general population, a control group of blood donors was set up, matched for age and sex. There was, however, no difference in HIV seroprevalence between the control group (9/735, 1.2%) and the leprosy group (1.5%). Although leprosy patients recruited for this study constitute a highly selected sample, it appears that HIV infection has little effect on leprosy, particularly on the PB/MB ratio, leprosy reactions and neuritis, but there is a suggestion the HIV infection might be associated with increased frequency of relapse.RÉSUMÉ
De février 1992 à juin 1994, tous les patients presentan! une lèpre prouvée à l'histopathologic, examines à l'Unité de la Lèpre à l'Institui Marchoux de Bamako, au Mali, out subi un examen sérologique pour le VIH. En tout, 740 malades de la lèpre ont été lestés; 553 cas connus et déjà traités antérieurement, et 187 cas nouveaux, principalement des cas qui s'étaient présentés d'eux-mêmes et des cas référés. La seroprévalence globale dans l'échantillon était de 1.5% (11/740), et a augmenté de 1.3% en 1992 à 3.1% en 1994. La seroprévalence pour le VIH était plus élevée pour les malades paucibacillaires (PB) que pour les malades mutibacillaires (MB) (3.8% contre 0.8%, p < 0.05), et était légèrement plus élevée parmi les nouveaux cas que parmi les cas connus et déjà traités antérieurement (2.1% contre 1.3%); cette différence n'était cependant pas significative. Parmi les 553 patients connus, déjà traités antérieurement, un des 7 patients séropositifs pour le VIH a présenté une récidive, par opposition à 34 parmi 546 patients séronégatifs (14.2% contre 6.2'/', p = 0.36). Parmi les nouveaux cas, aucun des 37 patients présentant une réaction et/ou une névrite n'était positif pour le VIH. Parmi les malades de la lèpre connus et traités, il n'y avait pas de différence dans la fréquence des réactions et/ou des névrites entre les cas positifs et négatifs pour le VIH. La migration vers un pays voisin est apparu être un facteur de risque de seropositivité pour le VIH dans notre échantillon (χ 2 = 4.5, p = 0.04). Afin d'estimer l'association du VIH et de la lèpre en comparaison avec la population générale, un groupe témoin de donneurs de sang a été établi, apparié pour l'âge et le sexe. Il n'y avait cependant pas de différence dans la seroprévalence vis-à-vis du VIH entre le groupe témoin (9/735, 1.2%) et le groupe de lépreux ( 1.5%). Bien que les malades de la lèpre recrutés pour cette étude constituent un échantillon fortement sélectionné, il apparaît que l'infection VIH a peu d'effet sur la lèpre, en particulier sur le ration PB/MB. les réactions lépreuses et la névrite, mais il est suggéré que l'infection VIH pourrait être associée à une plus grande fréquence de récidives.RESUMEN
Desde febrero de 1992 hasta junio de 1994 todos los pacientes con lepra probada histológicamente examinados en la Unidad de Leprología del Instituto Marchoux en Bamako, Malí, fueron estudiados para establecer su posible infección con HIV. En total se probaron 740 pacientes con lepra; 53 casos previamente conocidos y 187 casos nuevos. La prevalência global de HIV en la muestra fue del 1.5% (11/740), y aumentó de 1.3% en 1992 a 3.1'/,. en 1994. La seroprevalecia de HIV fue mayor en los casos paucibacilares (PB) que en los multibacilares (MB) (3.8% r.v 0.8%, p = 0.05) y fue ligeramente más alta en los casos nuevos que en los casos conocidos ya tratoados (2.1% r.v 1.3%). Entre los 553 casos ya conocidos y tratados sólo uno de los 7 casos HIV-seropositivos recayó, en contraste con 34 de los 546 casos HIVseronegativos (14.2% vs 6.2%, p = 0.36). Entre los casos nuevos ninguno de los 37 pacientes con reacción o neuritis fue 111 V-positivo. En los casos conocidos tratados no hubo diferencia en la frecuencia de reacciones o neuritis entre los casos HIV-positivos y los HIV-negativos. La migración a países vecinos pareció ser un factor de riesgo para la seropositividad a 11IV (χ2 - 4.5, p = 0.04). Para calcular la prevalência de HIV en los pacientes con lepra y en la población general, se incluyó un grupo control de donadores de sangre de la misma edad y mismo sexo. No hubo diferencia en la seroprevalencia de HIV entre el grupo contra (9/735, 1.2%) y el grupo de lepra (1.5%). Aunque los pacientes con lepra incluidos en este estudio constityeron una muestra altamente seleccionada, los resultados sugieren que la infección con HIV tiene poco efecto sobre la lepra, particularmente en cuanto a la relación PB/MB y la frecuancia de reacciones leprosas y neuritis, sin embargo se sugiere que la infección por HIV puede estar asociada a una incrementada frecuencia de recaídas.It is now well recognized that HIV infection constitutes a major risk factor for tuberculosis and for other mycobacteria, such as Mycobacterium avium-intracellulare (4, 9, 32), but there are still uncertainties regarding its association with leprosy. Potential effects of HIV infection on leprosy have been suggested and discussed by several authors (1, 7, 35) but, despite expectations, little interaction has been observed up to now (19). Although an association between HIV and leprosy has been described in Zambia (21) and in Tanzania (3), there is some evidence from studies in Malawi, in Ethiopia and in other African countries that HIV infection is not a risk factor for leprosy (11, l7, 30, 33). One of the reasons advanced is that, due to the very slow proliferation of the bacilli, clinical signs of leprosy would appear very late in the course of immunosuppression, and many individuals would die of other infections before leprosy declares itself (19, 30). Similarly, some authors raised the possibility of an increased incidence of relapses in already treated leprosy patients as a consequence of HIV infection (15, 26, 30), but this was not confirmed by others (11, 17).
It had been suggested that, through immunosuppression, HIV might enhance downgrading and shifts toward the lepromatous end of the leprosy spectrum, thus increasing the ratio of multibacillary (MB) to paucibacillary (PB) forms of leprosy (35). In fact, several studies in Africa, where the PB form of leprosy is more frequent than the MB form, did not confirm it (17, 30). Similarly, it was thought that, due to the fall in CD4 lymphocytes, HIV infection might increase the risk of type 2 reaction (35), but evidence is as yet missing (22). There have been reports of HIV-infected leprosy cases presenting a type 1 reaction (2, 27) but, since type 1 reaction is associated with variations in cell-mediated immunity (CMI) toward M. leprae antigens (14, 36), the effect of HIV infection on type 1 reactions still appears unclear (19, 22). Also, since damage to the peripheral nerves is a well known feature of infection with M. leprae, often associated with type 1 reaction (16, 18), there was some concern that HIV and M. leprae might enhance each other to cause more acute neuritis (25). A recent study in Uganda showed that HIV infection was associated with an increased incidence of type I reactions and neuritis among MB patients (6). In the Malawi study, however, all incident leprosy cases who had disability (consecutive to severe neuritis) were HIV negative (30), and in Ethiopia, there was no difference in the frequency of reactions between HIV-positive and HIV-negative leprosy cases (11).
In order to investigate the different effects of HIV infection on leprosy, we conducted a seroprevalence survey among leprosy patients examined at the Institut Marchoux in Bamako, Mali.
MATERIALS AND METHODS
From February 1992 to June 1994, a systematic HIV serology test was performed on all leprosy patients examined at the Leprology Unit of the Institut Marchoux. This Institute is a reference center for leprosy in Mali, receiving self-reporting and referred patients from the whole country. In addition, during the late 1970s-early 1980s, various clinical trials of rifampin-containing combined regimens have been carried out at the Institute, recruiting selectively MB patients. Therefore, patients recruited in this study are separated into two groups: a) the "already known" (or prevalent) leprosy cases (known cases, KC), who were treated for long periods with dapsone (DDS) and/or were recruited into clinical trials and continue to consult for long-term follow up, and b) the "new" or incident cases (new cases, NC), who are newly diagnosed patients, never treated before. At recruitment, patients underwent a complete examination, including inspection and palpation of skin and peripheral nerves. Nerve function was assessed through sensory testing with Nylon monofilaments and voluntary motor testing, using standard guidelines (37). In each suspected case, skin smears were taken from 4 sites (2 lesions and 2 earlobes) and a 4-mm punch biopsy was taken from an active skin lesion for confirmation of diagnosis and histological classification. PB and MB forms of leprosy were defined according lo the World Health Organization (WHO) standard guidelines (38).
Neuritis was defined as the presence of pain in the nerve(s), associated with enlargement and/or deterioration of nerve function (16). The diagnosis of type 1 reaction was suspected in the presence of inflammatory and/or raised, warm and infiltrated skin lesions, associated or not with fever and general signs (14). Type 1 reactions could be associated with neuritis. Type 2 reaction diagnosis was suggested if the patient presented the classic painful, inflammatory dermo-hypodermal nodules, associated or not with fever, general signs, and/or neuritis (14). Both type 1 and type 2 reactions were confirmed histologically. Relapse was suspected if a known treated case presented with one or more new skin lesion(s) and/or with a reactivation of old skin lesions. Diagnosis was confirmed if the bacterial index (BI) in the slit-skin smears had increased by at least 2 on the Ridley-Jopling scale (31), and/or if leprosy was confirmed histologically. In some cases, inoculation to mice was done.
Sera from all patients were separated from whole blood within 4 hr of collection, split and stored frozen until the day of analysis. Sera were first tested for HIV serology with an immuno-assay (Rapid HIV1-HIV2 AB; Clonatec, Paris, France) at the Institut Marchoux. Positive samples were confirmed by Western blot (WB) (New LAV blot 1 and 2; Pasteur Diagnostic). If a serum was undetermined with WB, a new blood sample was collected 3 months later and retested with an ELISA and WB.
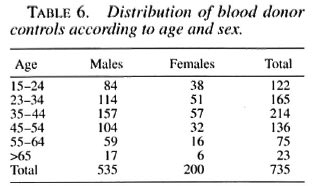
Although not planned originally, in order to estimate the association of HIV with leprosy as compared to the general population, a control group of blood donors was set up. In Bamako, blood donors are generally healthy persons from the family of a sick individual (medical or surgical condition) who needs blood transfusion. Controls were matched with the leprosy cases on age (5year groups) and sex, but not on residence, since data were insufficient.
RESULTS
In total, 740 leprosy patients have been tested for HIV seroloszy between February 1992 and June 1994; 553 known, previously treated cases and 187 newly diagnosed leprosy cases (Table 1). Among these patients, 584 were MB and 156 PB, with a large majority of MB cases among the known cases (χ 2 = 193, p < 0.001), due to the selected recruitment of these patients. Sex distribution was unbalanced, with 539 males (mean age 39.3) for 201 females (mean age 37.7). New cases were generally younger (mean age 30.7) than known cases (mean age 41.6). There were more males among new than among known cases.
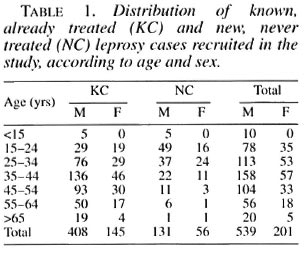
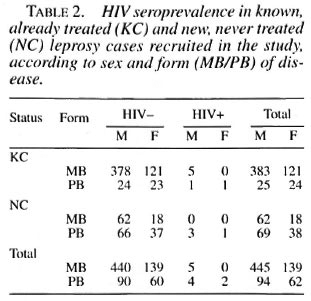
On the 740 patients tested with the ELISA rapid test, 27 (3.6%) were positive; after control with WB, 11(1.5%) were confirmed positive, 3 were negative and 13 undetermined. Among the last group, after a new control 3 months later, 9 became negative and 4 remained undetermined. Among the 11 HIV seropositive leprosy cases, there were 9 males and 2 females, with ages ranging from 21 to 45 (mean 33.7). Seven were known cases of leprosy (5 positive for HIV1 and 2 positive for HIV-2) and 4 were incident leprosy cases (all HIV-1 positive) (Table 2). There was no difference in seroprevalence between known and new cases after stratification on age (χ 2MH = 0.07, p = 0.7). HIV seroprevalence was, however, higher among PB (3.8%) than among MB cases (0.8%) after stratification on patients' status (known case/new case) ( χ 2MH = 6.2, p = 0.012), although there was a majority of MB patients among old cases. Seroprevalence was identical in 1992 and 1993 (1.4%), but doubled in 1994 (3.1%) although the numbers are quite small (Table 3). Clinically, among the 11 HIV-seroposi tive leprosy patients, 6 were asymptomatic, 1 had a proliferative generalized lymphadenopathy (stage I/II, CDC), 2 had signs and symptoms of ARC (WHO/Bangui classification), and 2 were clinically confirmed AIDS cases (Bangui classification).
 >
>
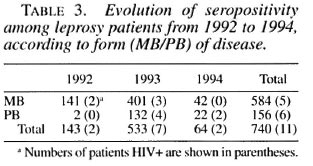
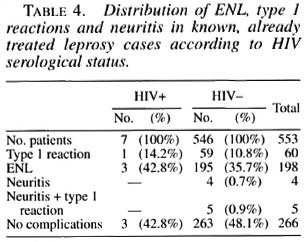
At the time of enrollment, 304/740 (41%) leprosy cases (267 known and 37 incident cases) presented with type 1 or type 2 reaction, associated or not with neuritis. There was no difference in HIV seroprevalence among leprosy cases in reaction (4/304, 1.3%) and those not in reaction (7/436, 1.6%). The four HIV-seropositive leprosy patients presenting with type 1 or type 2 reaction were asymptomatic regarding HIV infection. Among the 187 new cases, none of the 37 patients presenting with a reaction and/or neuritis was HIV positive. Among the 553 known, already treated cases, there was no difference in the frequency of type 1 or type 2 reaction between HIV-positive and HIV-negative cases (Fisher exact test p > 0.5) (Table 4). No neuritis case was diagnosed among HIV-positive leprosy patients.
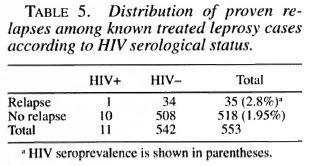
Thirty-five out of 553 known, previously treated leprosy cases presented at the Unit in a state of relapse, 1 among the 7 (14.2%) HIV positive and 34 among the 546 (6.2%) HIV negative. The frequency of relapses was thus slightly higher in HIV-positive than in HIV-negative leprosy cases, although not significantly (Fisher exact test, p = 0.4). We must note, however, that the type of disease, the type of previous treatment and the period elapsed since the end of the treatment were quite different among these cases.
Migration to a neighboring country (especially the Ivory Coast) appears significantly associated with an increased risk of HIV seropositivity in our sample: among leprosy cases who travelled to work abroad, HIV seroprevalence was higher (6/196, 3.1%) than among resident leprosy cases (5/544, 1%) ( χ 2 = 4.5, p = 0.04).
In order to compare with the general population, 735 blood donors, matched for age and sex, have been tested for HIV serology (Table 6). In this group, 11 individuals were positive with the rapid test. After control with WB, 9/735 (1.2%) were confirmed HIV positive ( 8 for HIV-1 and 1 for HIV-2) and 2 were seronegative. If we consider that blood donors represent the general population, HIV infection does not appear significantly associated with leprosy in our sample (Fisher exact test, p > 0.5), although HIV seroprevalence appears very low among both the leprosy cases and the controls.
DISCUSSION
The leprosy patients recruited in this study are referred cases and hospital patients, who are usually affected with a more severe disease than the general population, or may present with complications. These individuals thus constitute a highly selected sample, not representative of leprosy patients diagnosed "in the field." This selection is reflected in the age/sex imbalance of the sample: there are more males than females and more MB than PB cases, which is not the classical pattern in Africa (28). The excess MB forms among known treated cases probably reflects the fact that, in the past, the Institut Marchoux was the only place for leprosy treatment in Mali, and people coming from far away to receive long-term (5 years or more) treatment with DDS used to settle down near the Institute. It also reflects the past, active recruitment of highly bacilliferous MB patients in various clinical trials (20). The higher number of PB forms among new incident cases and their younger age reflect the improvement in the detection and treatment of leprosy by the national Leprosy Control Programme. Although there was no difference in the HIV-seroprevalence rate between known, treated cases and new, incident cases, the higher HIV seroprevalence in PB than in MB cases could reflect both the relatively recent introduction of HIV into the leprosy population and the migration of young PB cases, together with a change in patients' recruitment at the Institute. This aspect is, however, not in favor of a specific association between HIV and MB leprosy, conversely to what was suggested by some authors who thought that, since MB leprosy is associated with reduced cellular immunity (13), HIV-induced defective CMI would enhance downgrading from the PB to the MB forms of leprosy (35). Up to now, there is no indication from studies in Malawi, Ethiopia, and other African countries that HIV infection would induce such a shift (11, 17, 30). We are aware of only one controlled study, in Tanzania, in which the authors found that HIV infection was associated with the development of MB leprosy, although the concordance between clinical and bacteriological classification was not complete (3).
For similar reasons, HIV infection was expected to increase the possibility of relapse after treatment (15, 26, 35). In a small study in Kenya, there were 28% relapse/ treatment cases in HIV-positive individuals against 5% in HIV-negative cases, but definition of relapse was not given (24). In the Malawi study, the authors raised the possibility of an increased incidence of relapse as a consequence of HIV infection, although numbers were quite small (30). In our study, the frequency of relapse cases was slightly higher among HIV-positive than among HIV-negative treated leprosy cases, but the difference was not statistically significant. In addition, HIV-negative leprosy controls were not matched for age, sex, date of onset, type of disease or type of treatment (30). Therefore, further population-based study in areas where leprosy patients are well registered and regularly followed up would be needed on that issue.
There was no evidence of increased frequency of leprosy reactions and/or neuritis in association with HIV infection in our sample. Two main forms of reactional states have been described in leprosy: type 1 and type 2 reactions (14, 36). Type 1 reaction is thought to be associated with a delayed-type hypersensitivity (DTH) reaction to M. leprae antigens in patients with borderline leprosy whose immunological status is unstable (18). Although often clinically undistinguishable, two forms of type 1 reaction have been described, representing two different pathogenic pathways: a) the "upgrading" or reversal reaction, occurring mainly in borderline cases, associated with a rapid increase in the CMI response to M. leprae (especially the CD4+ lymphocyte response) and accompanied by a shift toward the tuberculoid end of the disease spectrum, and b) the "downgrading" reaction, associated with a partial loss of immunity and a shift toward the lepromatous pole (14, 18, 36). Since HIV infection induces a fall in CD4+ lymphocytes and has been associated with negative DTH, HIV-infected leprosy patients might be prone to develop a downgrading type 1 reaction (25, 35) rather than upgrading type 1 reaction. There have been some case reports of type 1 reaction in HIV-infected leprosy patients (2, 15). In Uganda, HIV infection has been reported to be associated with an increased incidence of type 1 reaction in MB leprosy patients (6), but type 1 reaction was not defined (neither clinically nor histologically) and it is not sure which form (downgrading or upgrading) was considered. In Ethiopia, the number of HIV-seropositive individuals was slightly higher among cases with reversal reaction (4/35, 11%) than in the entire group (31/644, 4.8%), although not significantly. Type 2 reaction, or erythema nodosum Ieprosum (ENL), occurs mainly in lepromatous leprosy, during and after treatment, and is thought to be primarily humoral. According to Turk and Rees, HIV-induced defective CMI might favor the occurrence of type 2 reactions in treated leprosy patients (35). In our study, there was no difference in the frequency of type 2 reactions (ENL) among HIV-positive and HIV-negative cases, although they were present mainly in known cases. In Ethiopia, there was no HIV seropositive patient among ENL cases (11). We are not aware of reports of ENL cases among treated MB leprosy patients with HIV infection (19, 22), which would be consistent with an involvement of CD4+ lymphocytes in ENL (23). The possible association of HIV infection and leprosy reactions thus raises questions about the immunological process at work in leprosy (10, 22).
Interaction of HIV and M. leprae at the level of peripheral nerves is a distinct possibility because peripheral neuropathy has been reported to be a neurological manifestation of HIV infection, in relation to the neurotrophicity of the virus (5). In addition to immunodeficiency, co-infected individuals may suffer from additional nerve damage, either due to concurrent reaction or to necrotizing vasculitis of the nerve due to HIV infection (17). In Uganda, HIV infection has been found to be associated with an increase in the incidence of neuritis and type 1 reaction in MB patients (p < 0.005), but it is not known whether the increased incidence of neuritis was due to type 1 reaction itself, to HIV infection, or to both (6). The possible interaction of M. leprae and HIV infection at the level of peripheral nerves would require further investigation, including nerve biopsy and virus culture.
We found a higher seropositivity rate among persons who travelled abroad than among resident leprosy cases, particularly for those travelling in Ivory Coast, where HIV prevalence in the general population is higher than in Mali (8). This is consistent with findings by Ponnighaus, et al ., who pointed out that "... Immigrant status is potentially an important confounder in circumstances in which the HIV seropositivity rate is different in a study population from that in surrounding populations." (30).
Our failure to observe an association between HIV infection and leprosy can be partly attributable to the selection of cases and controls. As we mentioned earlier, cases recruited in our study are not representative of all leprosy cases in a general population, since self-reported and referred cases tend to have more serious disease and present more complications than those originating from the general population. In addition, cases recruited in clinical trials 10 years ago were selected according to stringent criteria. Recruitment of blood donors as a control group is liable to bias since their relatives may have been hospitalized for a condition related to HIV infection. Lastly, if utilization of health services rather than the disease under study itself was associated with HIV infection, both groups would be biased, thus decreasing the chance of finding an association between HIV and leprosy. It has not been possible to match cases and controls for residence, which is a known confounder (30), and it is extremely probable that the two populations differ on that characteristic. In addition, in our sample HIV seroprevalence was low, which decreases the reliability of the estimates, and if a slight association did in fact exist, we were not able to detect it. Our results support, however, the absence of an association between HIV infection and leprosy already reported in several studies in Malawi, Ethiopia, and other African countries (11, 17, 30, 33). In particular, our results do not differ much from those found by Tounkara, et al. in a study in Bamako in 1989: among 210 leprosy patients, HIV seroprevalence was 3.8%; whereas in 160 blood donors, HIV seroprevalence was 3.12% (34). There is, thus, converging evidence that leprosy is little affected by HIV infection, probably due to the very slow development of leprosy, sometimes taking many years. Thus, HIV-infected individuals may die of other infections before clinical infection with M. leprae reveals itself (19, 30). However, the possibility of an increased incidence of relapses as a consequence of HIV infection still awaits further confirmation.
Apart from a hospital study in Zambia, which suffers from similar bias in the recruitment of cases and controls (21), we are aware of only one carefully controlled study in Tanzania, in which the overall odds ratio (OR) for the association between HIV-1 infection and leprosy was at the limit of statistical significance (OR = 2.2; CI = 1.0-4.7) (3). The significance of the association disappeared if two MB cases who did not have a positive slit-skin smear were reclassified as PB cases (OR = 3.6; 95% CI = 0.9-11.6). In that study, cases were passively detected and controls were chosen from a stratified cluster sample from urban areas, roadside settlements and rural villages. Among the controls, HIV seroprevalence was much higher in roadside and urban areas than in rural areas, showing a difference in the geographical distribution of the two infections, and the association between HIV-1 infection and leprosy was only apparent in the rural area and not in the urban area. This study illustrates quite well the difficulties in demonstrating an interaction between HIV infection and leprosy, due to: a) the difference in geographical distribution of the two infections (rural versus urban); b) the problem of concordance between clinical and bacteriological classification of leprosy and the difficulty in obtaining a reliable operational definition of leprosy (29) and c) the small and even decreasing number of new leprosy cases detected in most areas in the world today (10), which adds to the difficulty of conducting population studies. In any case, the study of the interaction of HIV infection and leprosy is limited by the impossibility to detect subclinical cases, thus causing us to rely on incident leprosy cases. If further studies on HIV and leprosy were to be done in areas where HIV has been present for a number of years, it would be interesting to associate lepromin and tuberculin testing and to evaluate the CD4+ lymphocyte count in HIV-positive and HIV-negative leprosy patients in order to understand better the course of the disease and to analyze its relationship to HIV-induced immunosuppression, especially with regard to reaction and neuritis (39).
Acknowledgment. This study has been possible through the financial support of the French Ministry of Cooperation (CDI 113/CD/92/314/04). The authors wish to thank all of the staff members of the Leprology Unit at the Institut Marchoux for their active contribution to this work.
REFERENCES
1. BASKIN, G. B., GORMUS, B. J., MARTIN, L. N., MURPHEY-CORH, WALSH, G. P. and MEYERS, W. M. Pathology of dual Mycobacterium leprae and simian immunodeficiency virus infection in rhesus monkeys. Int. J. Lepr. 58(1990)358-364.
2. BLUM, L., FLAGEUL, B., Sow, S., LAUNOIS, P., VIGNON-PENNAMEN, M.-D., COLL, A. and MILLAN, J. Leprosy reversal reaction in HIV-positive patients. Int. J. Lepr. 61(1993)214-217.
3. BORGDORF, M. W., VAN DEN BKOEK, J., CHUM, H. J., KLOKKE, A. H., GRAF, P., BARONGO, L. R. and NEWELL, J. N. HIV-1 infection as a risk factor for leprosy; a case control study in Tanzania. Int. J. Lepr. 61(1993)556-562.
4. BRAUN, M. M., BADI, N., RYDER, R. W., BAENDE, E., MUKADI, Y., NSUAMI, M., MATELS, B., WILLAME, J. C, KABOTO, M. and HEYWARD, W. A retrospective cohort study of the risk of tuberculosis among women of childbearing age with HIV infection in Zaire. Am. Rev. Respir. Dis. 143(1991)501-504.
5. BUDKA, H., WILEY, C. A., KLEIHUES, P., ARTIGAS, J., ASBURY. A. K., CHO, E. S., CORNBLATH, D. R., CAL CANTO, M. C, DEGIROLAMI, U., DICKSON, D., et al. HIV-associated disease of the nervous system: review of nomenclature and proposal for neuropathology-based terminology. Brain Pathol. 1(1991)143-152.
6. BWIRE, R. and KAWUMA H. J. S. Type I reactions in leprosy, neuritis and steroid therapy: the impact of the human immunodeficiency virus. Trans. R. Soc. Trop. Med. Hyg. 88(1994)315-316.
7. DAUMERIE, D., CASTELLI, F., PIZZOCOLO, G. and CAROSI, G. HIV and leprosy: a hypothetical mutual interference. (Abstract TH-78) 2nd Int. Symp. AIDS Africa, 1987.
8. DE COCK , K. M., PORTER, A., ODEHOURI, K., BARRERE, B., MOREAU, J., DlABY, L., KOUADIO, J. C. and HEYWARD, W. L. Rapid emergence of AIDS in Abidjan, Ivory Coast. Lancet 2(1989)408-411.
9. DE COCK, K. M., SORO, B., COULIBALY, I. M. and LUCAS, S. B. Tuberculosis and HIV infection in sub-Saharan Africa. JAMA 268(1992)1581-1587.
10. FINE, P. E. M. Reflections on the elimination of leprosy. (Editorial) Int. J. Lepr. 60(1992)71-80.
11. F ROMMEL, D., TEKLE-HAIMANOT, R., VERDIER, M., NEGESSE Y., BULTO, T. and DENIS, F. HIV infection and leprosy: a four-year survey in Ethiopia. Lancet 344(1994)165-166.
12. GHERARDI, R., LEBARGY, F., GAULARD, P., MHIRI, C. and BERNAUDIN, J. F. Necrotizing vascultits and HIV replication in peripheral nerves. N. Engl. J. Med. 321(1989)685-686.
13. HARBOE, M. The immunology of leprosy. In: Leprosy. Hastings, R. C , ed. Edinburgh: Churchill Livingstone, 1985, pp. 88-89.
14. HASTINGS, R. C, ED. Leprosy. Edinburgh: Churchill Livingstone, 1985.
15. JANSSEN, F, WALLACH, D., KHUONG, M. A., PENNEC, M. A., PRADINAUD, R. and COTTENOT, F. Association de maladie de Hansen et d'infection par le virus de l'immunodeficience humaine; deux observations. Presse Med. 17(1988)1652-1653.
16. Jon, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
17. LEONARD, G., SANGARE, A., VERDIER, M., SASSOU-GUESSEAU, E., PETIT, J., MILIAN, J., M'BOUP, S., REY, J. L., DUMAS, J. L., HUGON, J., N'GAPOGO I. and DENIS, F. Prevalence of HIV infection among patients with leprosy in African countries and Yemen. J. Acquir. Immune Defic. Syndr. 3(1990)1109-1113.
18. LIENHARDT, C. and FINE, P. E. M. Type I reaction, neuritis and disability in leprosy. What is the current epidemiological situation? Lepr. Rev. 65(1994)9-33.
19. LUCAS, S. Human immunodeficiency virus and leprosy. (Editorial) Lepr. Rev. 64(1993)97-103.
20. MARCHOUX CHEMOTHERAPY STUDY GROUP. Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
21. MEERAN, K. Prevalence of HIV infection among patients with leprosy and tuberculosis in rural Zambia. Br. Med. J. 298(1989)364-365.
22. MILLER, R. A. Leprosy and AIDS: a review of the literature and speculations on the impact of CD4+ lymphocyte depletion on immunity to Mycobacterium leprae. Int. J. Lepr. 59(1991)639-644.
23. MILLER, R. A., SHEN, Y.-Y., REA, T. H. and HARNISCH, J. P. Treatment of chronic erythema nodosum leprosum with cyclosporine A produced clinical and immunohistologic remission. Int. J. Lepr. 55(1987)441-449.
24. OREGE, P. A., ODAWA, B. A., OKELLO, C. M., OBURA, M., OKUKU, P., AMIMO, R. K. and WERE, M. The effect of HIV infection on clinical response of leprosy patients to multidrug therapy in Kenya. (Abstract) Int. J. Lepr. 61 Suppl. (1993)36A.
25. PATKI, H. Some possible interactions of M. leprae and HIV in the peripheral nerves. Int. J. Lepr. 59(1991)331-332.
26. PEAN, C, PAPE, J. W., DESCHAMPS, M. M. and DAMBREVILLE, M. Prévalence et évolution de l'infection au virus humain d'immunodélicience (VIH) chez les lépreux en Haiti. (Abstract) Int. J. Lepr. 57 Suppl. S306-S307.
27. PEAN, C, PAPE, J. W., DESCHAMPS, M. M. DAMBREVILLE, M. and JOHNSON, W. D. Natural hsitory of M. leprae and HIV infection. (Abstract BP70) In: Proceedings of the V International Conference on AIDS, Montreal, 4-9 June 1989. Ottawa: International Developmental Research Centre, 1989, p. 427.
28. PÖNNIGHAUS, J. M. and FINE, P. E. M. Sensitivity and specificity of the diagnosis and the search for risk factors from leprosy. Trans. R. Soc. Trop. Med. Hyg. 82(1988)803-809.
29. PÖNNIGHAUS, J. M., FINE, P. E. M., MAINE, N., BLISS, L., KALAMBO, M. and PÖNNIGHAUS, I. The Lepra Evaluation Project (LEP), an epidemiological study of leprosy in northern Malawi. II. Prevalence rates. Lepr. Rev. 59(1988)97-112.
30. PÖNNIGHAUS, J. M., MWANJASI, L. J., FINE, P. E. M., SHAW, M. A., TURNER, A. C, OXBORROW, S. M., LUCAS, S. B., JENKINS, P. A., STERNE, J. A. C. and BLISS, L. IS HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
31. REES, R. J. W. The microbiology of leprosy. In: Leprosy. Hastings, R. C, ed. Edinburgh: Churchill Livingstone, 1985, pp. 31-52.
32. SELWYN, P. A., HARTEL, S. and LEWIS, J. A. A prospective study of the riske of tuberculosis among intravenous drug users with HIV infection. N. Engl. J. Med. 320(1989)545-550.
33. TEKLE-HAIMANOT, R., F ROMMEL, D., TADESSE, T, VERDIER, M., ABEBE, M. and DENIS, F. A survey of HTLV-I and HIVs in Ethiopian leprosy patients. AIDS 5(1991)108-110.
34. TOUNKARA, A., FOPANA, Y. DIABATE, M. and SANGARE, D. Etude de la seroconversion anti VIH chez les lépreux lepromateux au Mali (enquête préliminaire). Méd. Afr. Noire 38(1991)89-91.
35. TURK, J.L., and REES, R. J. W. AIDS and leprosy. Lepr. Rev. 59(1988)193-194.
36. WATERS, M. F. R, TURK, J. L., and WEMAMBU, S. N. C. Mechanisms of reactions in leprosy. Int. J. Lepr. 39(1971)417-428.
37. WATSON, J. M. Disability control in a leprosy control programme. Lepr. Rev. 60(1989)169-172.
38. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
39. World Health Organization. Report of a meeting on HIV infection in leprosy. Int. J. Lepr. 61(1993)642-643.