- Volume 64 , Number 4
- Page: 449–51
M. leprae and macrophage secretory products modulate the expression of NgCAM on Schwann cell surface
To the Editor:
During embryogenesis and under pathological conditions, Schwann cells (SC) of the peripheral nerve are induced to express cell-surface molecules, such as the neuroglial cell adhesion molecule (NgCAM), which aid in the initial SC-axon associations needed for axon fasciculation (5). Infection with Mycobacterium leprae of its host cell, the macrophages, renders them defective in a number of functions, including the expression of cell-surface molecules (1). The possibility exists that SCs, for which M. leprae have a special affinity, also could be rendered defective in the expression of cell-surface molecules and, therefore, contribute to the peripheral nerve pathology in leprosy. The presence of features like aberrant myelination in the sciatic nerve of the murine animal model inoculated with M. leprae in the foot pad (7) may indicate variation in adhesion molecule expression. Besides this, macrophages which infiltrate the site of a nerve lesion in order to aid SCs in nerve regeneration (6), secrete a host of cytokines which have been shown to regulate the expression of cell-adhesion molecules (4).
The aim of this study was, therefore, to determine if M. leprae infection and macrophage secretory products modulate the expression of NgCAM on the SC surface, comparing the differences in cells derived from two strains of mice, Swiss white (SW) and C57BL/6 mice, in their response to M. leprae infection (2). (The viable M. leprae used in our study was derived from frozen armadillo liver biopsies supplied by Dr. E. Storrs, Florida Institute of Technology, Melbourne, Florida, U.S.A.)
Dissociated Schwann cells (DSC) from the sciatic and brachial nerves of 1-2-dayold mice were cultured on coverslips in a 10% feeding medium (FM) essentially by the method of Brockes, et al. (3), Five-dayold cultures were infected with M. leprae (5 x 108ml) for 24 hr following which the excess bacilli were washed off. Conditioned medium from cultures of peritoneal macrophages was added to uninfected and 3-day postinfected DSC cultures with FM in a ratio of 1:1 for 72 hr. NgCAM expression was determined by indirect immunofluorescence staining as follows: Cells were-fixed with 70% ethanol and incubated with a 1:50 dilution of rabbit polyclonal antibody to human brain NgCAM and a 1:200 dilution of FITC-labeled anti-rabbit IgGs. The cells were mounted on PBS-glycerol and observed under a fluorescence microscope.
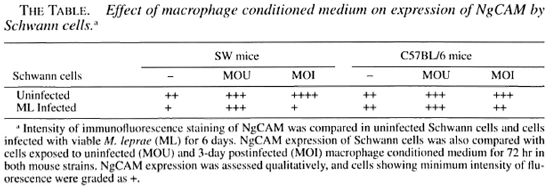
The NgCAM was expressed by almost all of the SCs, and the few fibroblasts present in the culture were negative. NgCAM was expressed to an equal extent by SCs from both strains of mice. However, following M. leprae infection, the expression decreased in intensity in SCs from SW mice. Uninfected SCs showed enhancement in NgCAM expression in the presence of both uninfected and M. leprae- infected macrophage conditioned medium. However, NgCAM expression of infected SCs was enhanced only in the presence of uninfected macrophage conditioned medium, especially SW SCs in which the M. leprae infection had initially decreased the NgCAM expression (The Table).
This indicates that in vivo regeneration attempts in an early leprous nerve could be hampered, in combination with other factors, by aberration in the SC association with the axons due to decrease in NgCAM expression following parasitization with M. leprae. But this does not take into consideration the status of axonal NgCAM expression which could be, alternatively, over- or under-expressed in a leprous nerve, thereby further influencing SC-axon associations. In addition, variation in response to infection in the two mouse strains also implicates a role for NgCAM in the divergence observed in the sciatic nerve pathology in foot pad-inoculated mice, with early comparable pathology in SW and C57BL/6 progressing to extensive demyelination only in SW mice (2). On the other hand, an influx of macrophages in the leprous nerve would enhance the expression of NgCAM and thus aid SCs in nerve regeneration. The fact that nerve damage still ensues in spite of a high macrophage population in the leprous nerve indicates the involvement of other complex mechanisms.
The infection of macrophages by M. leprae results in the downregulation of a number of cell-surface receptors (1). Similarly, invasion by M. leprae seems to result in membrane perturbation of the SC surface also, as indicated by the decrease in NgCAM expression. NgCAM may be just one of a number of other SC surface molecules to be altered on M. leprae invasion, and these changes in conjunction with one another may contribute to the leprous nerve pathology.
- Neeta Singh, M.Sc.
Research Student
- Tannaz J. Birdi, Ph.D.
Senior Research Officer
- Noshir H. Antia, F.R.C.S.,
F.A.C.S.(Hon.)
Director and Trustee
The Foundation for Medical Research
84-A R.G. Thadani Marg
Worli, Bombay 400 018, India
Acknowledgment. We thank Dr. Elizabeth Bock. University of Copenhagen, for generously providing us with the anti-NgCAM antibodies and Dr. Nergis Mistry for her assistance in the study. This work was funded by grant No. 030074/Z/89/z. from The Wellcome Trust, London, U.K. N. Singh was a recipient of a fellowship from the Council of Scientific and Industrial Research. Government of India.
REFERENCES
1. BIRDI, T. J. and ANTIA, N. H. The macrophage in leprosy: a review on the current status. Int. J. Lepr. 57(1989)511-525.
2. BIRDI, T. J., SHETTY, V. P, and ANTIA, N. H. Difference in M. leprae -induced nerve damage in Swiss white and C57BL/6 mice. (Letter) Int. J. Lepr. 63(1995)573-574.
3. BROCKES, J. P., FIELDS, L. K. and RAFF, M. C. Studies on cultured rat Schwann cells I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 165(1979)105-118.
4. EINHEBER, S., HANNOCKS, M., METZ, C. N., RIFKIN, D. B. and SALZER, J. L. Transforming growth factor- 1β regulates axon/Schwann cell interactions. J. Cell Biol. 129(1995)443-458.
5. MARTINI, R. and SCHACHNER, M. Immunoelectron microscopic localisation of neural cell adhesion molecules (L1, N-CAM and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J. Cell Biol. 106(1988)1735-1746.
6. PERRY, V. H., BROWN, M. C. and GORDON, S. Macrophage response to central and peripheral nerve injury; possible role for macrophages in regeneration. J. Exp. Med. 166(1987)1685-1701.
7. SHETTY, V. P. and ANTIA, N. H. Myelination around multiple axons in the peripheral nerve: an unusual ultrastructural observation. Neuropathology 50(1980)147-151.
Reprint requests to Dr. Birdi.