- Volume 64 , Number 4
- Page: 433–40
Electrophysiological evaluation of peripheral autonomic function in leprosy patients, leprosy contacts and controls
ABSTRACT
Since there is immunocytochemical evidence that the initial damage in leprosy is directed at distal, small, unmyelinated nerve fibers, we investigated several electrophysiological methods for their potential value in detecting peripheral autonomic dysfunction in leprosy contacts and leprosy patients. Fingertip blood flow velocity and its control by vasomotor reflexes (VMR) with a laser Doppler flowmeter, fingertip skin temperature, and the sympathetic skin response (SSR) to exosomatic stimuli were studied in 89 leprosy patients, 36 leprosy contacts and 47 normal subjects. Whereas there were no significant differences between the groups in fingertip skin temperature and resting blood flow velocity measurements, there were significant differences in the prevalence of impaired fingertip VMR and absent SSR. The prevalence of absent SSR in leprosy patients was 60.9%, in contacts 13.8%, in controls 6.3%. The prevalence of abnormal VMR in leprosy patients was 61.2%, in contacts 34.7% and in controls 10.6%. VMR testing is a more sensitive test method for autonomic dysfunction compared with the SSR. The implication of impairment in vasomotor and sudomotor function in leprosy contacts needs yet to be determined. However, we propose this to be a response to exposure to Mycobacterium leprae, which represents either ongoing nerve damage or nonprogressive residual autonomic nerve damage. We suggest that VMR testing and SSR are valuable methods to evaluate early leprous neuropathy.RÉSUMÉ
Depuis qu'il y a des preuves immunocytochimiques que la lésion initiale de la lèpre est située au niveau de petites libres nerveuses distales non myélinisées, nous avons étudié différentes méthodes électrophysiologiques quant à leur valeur potentielle pour détecter un dysfonctionnement autonomique périphérique chez des contacts de lépreux et des malades de la lèpre. La vitesse du flux sanguin au bout du doigt et son contrôle par réflexes vaso-moteurs (RVM), mesurés par un appareil de Doppler à laser, la température de la peau au bout du doigt, et la réponse cutanée sympathique (RCS) à des stimuli externes ont été étudié chez. 89 malades de la lèpre, 36 contacts de lépreux, et 47 sujets normaux. Alors qu'il n'y avait pas de différences significatives entre les groupes en ce qui concerne la température cutanée au bout des doigts et les mesures de vélocité du flux sanguin au repos, il y avait des différences significatives dans la prévalence de RVM altérés au bout des doigts et l'absence de RCS. La prévalence de l'absence de RCS était de 60.9% chez les malades de la lèpre, de 13.8% chez, les contacts et de 6.3% ches les témoins. La prévalence de RVM anormaux était de 61.2% chez les malades de la lèpre, 34.7% chez, les contacts et 10.6% chez les témoins. Le test RVM est une méthode plus sensible pour le dysfonctionnement autonomique, comparé à la RCS. Les implications d'une altération des fonctions vasomotrice et sudomotrice chez des contacts de malades de la lèpre doivent encore être déterminées. Cependant, nous proposons l'hypothèse que ceci soit une réponse à l'exposition au Mycobacterium leprae et représente soit une altération nerveuse progressive, soit des dégâts résiduels non progressifs des nerfs autonomes. Nous suggérons que les tests des RVM sont des méthodes de valeur pour rechercher la présence d'une neuropathic lépreuse précoce.RESUMEN
Debido a que hay evidencias inmunocitoquímicas de que el daño inicial en la lepra está dirigido a las pequeñas libras nerviosas distales no mielini/.adas, en este trabajo investigamos la utilidad de varios métodos electrotisiológicos para detectar la disfunción autónoma periférica en los pacientes con lepra y en sus contactos. Se midieron la velocidad de flujo sanguíneo en la punta de los dedos y su control por reflejos vasomotores (VMR) con el flujómetro de Doppler, la temperatura de la piel en la punta de los dedos, y la respuesta simpática de la piel (SSR) a estímulos externos en 89 pacientes con lepra, en 36 de sus contactos y en 47 individuos sanos. Mientras que no hubieron diferencias significativas entre los grupos en cuanto a la temperatura de la piel de la punta de los dedos y a las mediciones de la velocidad de flujo sanguíneo en reposo, sí hubieron diferencias significativas en cuanto a la ausencia de SSR y a los defectos en sus VMR. El grado de ausencia de SSR fue del 60.9% en los pacientes, del 13.8% en sus contactos, y del 6.3% en los controles. La medición de los VMR es una prueba más sensible que la medición de la SSR para establecer la disfunción autónoma. La posibilidad de defectos en la función vasomotora y pseudomotora en los contactos de los pacientes con lepra es un aspecto que debe analizarse con más detalle. Sin embargo, creemos que nuestros resultados podrían indicar un daño nervioso en evolución (como consecuencia de la infección con Mycobacterium leprae) o un daño residual no progresivo de los nervios autónomos. Sugerimos que las mediciones de los VMR y de la SSR son útiles para evaluar la neuropatía temprana de la lepra.Mycobacterium leprae not only affects somatic motor and sensory nerves but also autonomic nerve fibers (16-18). Both immunocytochemical (8) and neurophysiological studies (2, 21) suggest that peripheral autonomic nerve fiber involvement may be the focus of the initial nerve damage in leprosy. Recent neurophysiological methods (2,21) have used vasomotor reflex Doppler velocimetry. This approach to the detection of neuropathic autonomic abnormalities was proposed by Low, et al. (11) and measures the transient fall in fingertip blood flow after an autonomic nerve stimulus such as the inspiratory gasp. There is a high prevalence of abnormal vasomotor reflexes (VMR) in leprosy patients (2,4,21).
In another study we have shown that contacts of leprosy patients also have evidence of abnormal VMR (Wilder-Smith, submitted). Autonomic dysfunction in leprosy contacts might indicate either very early neuropathy or nonprogressive residual damage following exposure to M. leprae.
In this study, we investigate and compare additional electrophysiological methods to support evidence of autonomous dysfunction in leprosy patients and contacts. Additional methods include the sympathetic skin response (SSR), fingertip skin temperature and resting skin blood flow.
The SSR is a reliable test of sympathetic sudomotor function (3,15). The SSR measures the changes in voltage of the skin in response to exosomatic stimuli. Fingertip skin temperature and resting blood flow are influenced by sympathetic vasoconstrictor reflexes of arteriovenous anastomoses in the deep dermis, and are thus an indirect indicator of autonomic function.
This paper compares parameters of autonomic function in leprosy patients and leprosy contacts using VMR laser Doppler velocimetry, SSR, fingertip skin temperature and blood flow to assess the potential value of these electrophysiological methods in the diagnosis of early leprosy neuropathy.
MATERIALS AND METHODS
Subject selection
Leprosy patients. The study was conducted at Green Pastures Hospital, Pokhara, in western Nepal from April to June 1995. A detailed description of patient selection is presented elsewhere (21). Patients with confirmed leprosy between the ages of 10 and 55 were included. Excluded were patients with diabetes mellitus, alcoholism, or any other polyneuropathies attributable to other causes such as leprosy, as well as patients with more than one digit missing or more than one finger pulp with total reabsorption, and patients who had undergone surgery on more than one limb.
Controls. Healthy controls were recruited from the local Red Cross Society as well as from friends and relatives of workers in the leprosy hospital. All controls had no known contact with leprosy.
Contacts. Healthy contacts were recruited from among relatives of patients living in the same house for at least 1 year prior to treatment or from health care professionals working in the leprosy hospital for more than 5 years.
The study was approved by the ethics committee in Bern, Switzerland, and by the local ethics committee in Pokhara, Nepal.
Neurophysiological testing
SSR. The SSR was performed as previously described (19). Briefly, surface electrodes were attached to the palm and dorsum of either hand as well as to the sole and dorsum of either foot after cleaning with an alcoholic solution. Single square pulses of 20 0 msec were delivered to the skin of the wrist and ankle. If the skin in that area was anesthetic, the pulses were applied to a normally sensitive skin area more proximally. The response was considered absent if no consistent voltage change, using a sensitivity of 50 mV/cm, was observed after at least 10 trials separated by long intervals to avoid the natural habituation of the response.
VMR testing. VMR testing has been reviewed in detail (11,21). Briefly, the VMR of all 10 fingertips and the big toes were measured. Blood flow was measured using a laser Doppler flow temperature monitor (Model DRT4; Moor Instruments, Axminster, U.K.). With ambient temperatures above 26.5ºC (mean 30.5ºC, maximum 34.5ºC) maximal vasodilatation was obtained. A double-sided adhesive disk attached the laser Doppler and temperature sensor probe to the fingertip pulp. To elicit the VMR a deep and sudden inspiratory gasp was used. The procedure was explained in Nepali and practiced before testing was begun. First, a stable baseline blood flow was recorded. An event marker marked the onset of each inspiratory gasp. The ensuing reduction in capillary flow was recorded and expressed as a percentage of the resting flow. In each subject the inspiratory gasp was repeated three times and the maximal response was taken for recording. Of all 12 measurement sites, the fall in the baseline blood flow in percentage was recorded.
Based on a previous study (21) we defined the cut-off values for an abnormal VMR at a threshold value of 1.96 S.D. from the corresponding mean value obtained in controls. This represented values of approximately 55% reduction of flux for the hands and 45% for the feet. Since we intended to correlate the result of one limb with the result of the SSR of the correspondent limb, we used the mean of the percentage blood flow drop of all five digits and defined that limb as abnormal if lower than 55% (for hands) or lower than 45% (for feet). We also analyzed the data using the criteria as suggested by Low, et al. (11). According to their criteria, an abnormal response is a decrease in blood How of less than the 5th percentile. The VMR of more than one digit has to be abnormal to define that limb as abnormal.
Measurement of skin temperature. The probe head of a platinum skin thermistor is built into the same unit as the laser Doppler velocimeter. It was attached to the pulp of the fingertip with double-sided adhesive tape. A stable temperature was generally achieved after 4 min of contact with the skin. The same sensor was used to measure atmospheric temperature.
Measurement of skin blood flow. The probe holder of a laser Doppler velocimeter was attached to the pulp of the fingertip with double-sided adhesive tape; 5 min were allowed to equilibrate at an ambient temperature of 27ºC-34ºC.
Statistical analysis. Data entry and statistical analysis were performed on EPI¬ INFO Version 6 (Centers for Disease Control and Prevention, Atlanta, Georgia, U.S.A.) and SAS Version 6.08 (SAS Inc., Cary, North Carolina, U.S.A.). Correlation between the SSR and VMR was performed with the Student's / test. For the comparison of baseline blood flow and the fingertip skin temperature, the Mann-Whitney test was used.
RESULTS
Patient characteristics
In the final analysis there were 89 leprosy patients, 36 contacts and 47 controls, for a total of 172 participants. The mean age was 35.0 years (range 11 to 55) among patients, 29.4 years (range 11 to 54) among contacts, and 30.2 years (range 10 to 55) among controls (p = 0.013 by analysis of variance). Male participants dominated all groups (74% male participants among leprosy patients, 64% among contacts, and 68% among controls; p = 0.5 by chi-squared test). Borderline tuberculoid was the most common type of leprosy (42%).
A complete set of 12 measurements for VMR testing was performed in all but three controls because of technical reasons. Also for technical reasons, three SSR results are missing in leprosy patients.
SSR. The prevalence of absent SSR looking at all limbs in leprosy cases was 60.9%, in contacts 13.8%. In controls it was 6.3%, with all negative SSR occurring only in the feet. In all groups the prevalence of absent SSR was significantly higher (p < 0.001, using the Student's t test) in the feet than in the hands. A summary of the results (separated into hands and feet) is given in Table 1. The difference between leprosy patients and controls for the prevalence of abnormal VMR and absent SSR was highly significant (p < 0.001); the difference between contacts and controls also was significant (p = 0.022).

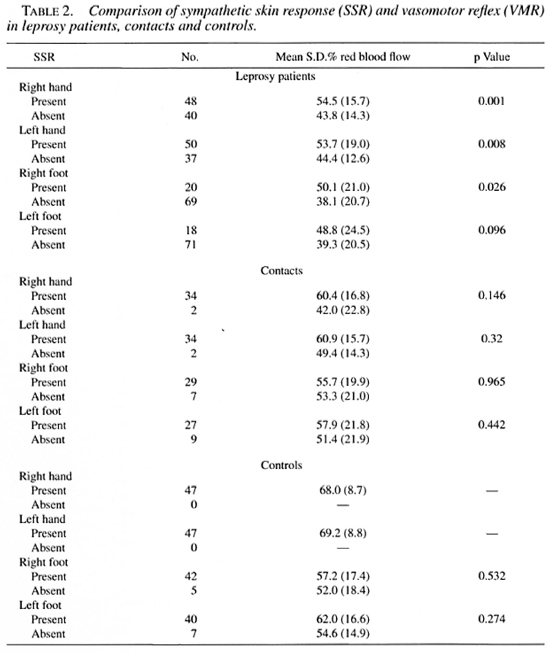
Correlation of SSR and VMR. Mean percent reductions of blood flow for the different measurement sites and groups compared to the sudomotor reflex are shown in Table 2. The mean percent reduction was statistically significantly lower for each limb in leprosy patients who had an absent SSR except for the left foot. Neither of the differences for controls or contacts were statistically significant.
Based on our previous study (21), we defined the cut-off values for an abnormal VMR at 1.96 S.D. of the corresponding mean measured in controls. This is approximately below 55% for hands and 45% for feet. Using this definition, there was a prevalence of 61.2% of abnormal VMR in leprosy patients, 34.7% in contacts, and 10.6% in controls.
However, we also analyzed the data using the criteria as suggested by Low, et al. (11). Accordingly, the VMR of more than one digit has to be abnormal to define that limb as abnormal. Abnormal is defined as lower than the 5th percentile which, using our data, is 39% for the left hand and 39.9% in the right hand. Table 3 compares the results using both criteria for the hands.
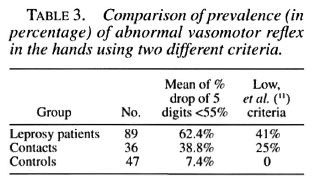
Fingertip skin temperature. There are no clear differences between any of the groups, but minimum temperature is always lowest in the leprosy patients. Table 4 shows the results of the fingertip skin temperature in leprosy patients, contacts and controls.
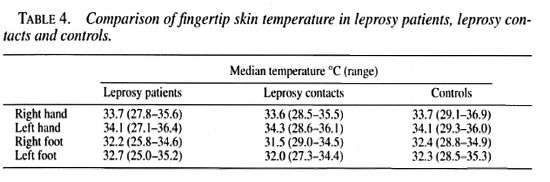
Resting fingertip blood flow. The baseline blood flow is significantly lower in leprosy patients compared with controls (except for the left foot). There are no statistically significant differences between leprosy contacts and controls. Table 5 shows the results of blood flow in all three groups.
DISCUSSION
Our data show significantly different prevalences of pathological SSR and VMR across the three groups of leprosy patients, leprosy contacts and controls. There is a significant concordance of SSR and VMR testing in leprosy patients. Although there is a significantly reduced blood flow in leprosy patients compared with controls, there is no difference between contacts and controls. Fingertip skin temperature was similar in all three groups, although the lowest temperature was always recorded in leprosy patients.
Since the earliest changes in teased nerve preparations affect the unmyelinated fibers(18), the sympathetic efferent pathway could well be the first site of attack by M. leprae. This would account for the higher prevalence of autonomic dysfunction in leprosy patients (61.7% for VMR, 60.8% for SSR) compared to the prevalence of sensory and motor impairment (33% had sensory and/or motor impairment at first examination in a study also performed at Green Pastures Hospital20). The significant impairment in blood flow in leprosy patients could be due to a resulting imbalance of the vasomotor afferences and efferences. In advanced leprosy damage to blood vessels may take place (1,5). Due to our patient selection criteria (no finger or, at most, only one finger missing), most had been diagnosed within the last 12 months (47% < 12 months, 20% > 6 years). For this reason it seems unlikely that direct vessel damage would contribute to resting blood flow reduction. An analysis of the distribution and prevalence of dysautonomia within leprosy subgroups compared to clinical severity of disease will be published elsewhere. The objective of this study was to determine any differences in autonomic testing within the groups to investigate their potential value in the detection of early or subclinical leprous neuropathy.
The overall prevalence of pathological VMR in contacts is 34.6% and of absent SSR 13.8%, significantly higher than in the control group. Blood flow and fingertip skin temperature are normal in contacts. The clinical relevance of impairment of fingertip VMR and SSR in the individual, apparently healthy, leprosy contact needs further evaluation. However, knowledge about autonomic dysfunction in leprosy contacts might help to unravel more about the pathogenesis and natural course of leprosy. If leprosy neuropathy is usually a progressive condition (7), the impairment in autonomic function in contacts might indicate pre-symptomatic disease. However, epidemiological data show (13) that the risk of developing disease in contacts is fairly low. In an 8-year study in India, the attack rate among contacts was 6.8/1000 (14). Therefore, it is more likely that electrophysiological evidence of autonomic dysfunction might present an immune tolerance reaction to the exposure to M. leprae with consequent immune-mediated damage to the small unmyelinated autonomic fibers.
The prevalence of abnormal VMR in the hands is higher than the prevalence of abnormal SSR. Karanth's immunocytochemical study (8) specifically looked at the differing extent of autonomic nerve damage innervating various skin organs. The group found that autonomic nerve fibers supplying blood vessels were more frequently damaged than those innervating sweat glands in patients with tuberculoid leprosy (42% of our leprosy patients suffered borderline tuberculoid leprosy). These observations may help to explain the higher prevalence of abnormal VMR compared to SSR. Furthermore, VMR tests autonomic function more focally and uses five measurements per hand compared to one for SSR. Since SSR is the product of a large sum of sweat glands distributed over the whole palm, a few nonfunctioning sweat glands will not be detected. These are the reasons for VMR's greater sensitivity in testing for dysautonomia (10). However, the higher rate of abnormal VMR compared to SSR in controls suggests that VMR testing has a lower specificity. In a study on a larger number of normal control subjects (11) as much as 21% had one abnormal test result, but no control had more than one abnormal response in either hand or foot, suggesting the following definition for an abnormal profile: 1) more than one absent respouse in the linger or the lingers or the toes, 2) reduction in response below the 5th percentile.
Using these criteria none of our controls had an abnormal profile in their hands. Since we only measured the big toe in the foot, these criteria were not applicable to the feet in our study. The disadvantage of VMR is that the testing is more time consuming and requires more examiner expertise and more compliance by the examinee.
Our results show a strikingly higher prevalence of absent SSR in the feet compared to the hands in all three groups. This may be due to the epidermal component of the SSR which is affected by skin thickness (6). Since Nepali subjects tend to have thicker soles due to frequent barefoot walking, this may well lead to false absent SSR. In fact, the control subjects never had an absent SSR in their hands. The advantage of the SSR is its simplicity; its disadvantage, the tendency to habituate (19).
Both reflexes have complex mechanisms (9), their end organ response is unmyelinated peripheral sympathetic fibers with two different components. Sudomotor and vasomotor components are both capable of independent responsivity to different stimuli (3). Thus, one must be careful not to rely on one test for dysautonomia alone for prognostication or therapeutic decisions. For further scientific purposes, the concurrent use of both or even additional methods would be advisable to determine and to evaluate different aspects of autonomous function in leprosy patients and in leprosy contacts.
Our results imply that VMR and SSR are valuable methods for testing for early or subclinical leprous neuropathy, and measuring fingertip skin temperature and resting blood flow are not.
Acknowledgment. The authors wish to acknowledge the valuable statistical help of Jo Morris, a biostatistician at the London Institute of Tropical Medicine and Hygiene. We are grateful for the financial support from the Swiss Academy of Medical Sciences and Sandoz. We are indebted to the staff of the physiotherapy department at Green Pastures Hospital (GPH) for their valuable practical help. Without the support of Dr. van Brakel, project manager of the Leprosy Control Project, Pokhara, Nepal, and Dr. Frauke Worpel, Superintendent of GPH, and Dr. Val Inchley. Medical Project Director of the International Nepal Fellowship, this work would not have been possible. The work of GPH for leprosy patients was initiated following Jesus Christ's example of Mark 1,41: "Jesus, moved with compassion, put out His hand and touched the leper."
REFERENCES
1. ABBOT, N. C, BECK, J. S., SAMSON, P. D., BUTLIN, C. R.. BENNETT, P. J. and GRANGE, J. M. Cold fingers in leprosy. Int. J. Lepr. 60(1992)580-586.
2. ABBOT, N. C, BECK, J. S., SAMSON, P. D., BUTLIN, C. R., BROWN. A. R., FORSTER, A., GRANGE;, J. M. and CREE, I. A. Impairment of fingertip vasomotor reflexes in leprosy patients and apparenty healthy contacts. Int. J. Lepr. 59(1991)537-547.
3. ARUNODAYA, G. R. and TALY, A. B. Sympathetic skin response: a decade later. J. Neurolog. Sci. 129(1995)81-89.
4. BECK, J. S., ABBOT, N. C, SAMSON, P. D, BUTLIN, C. R., GRANGE, J. M., CREE, L A., FORSTER, A. and KHAN, F. Impairment of vasomotor reflexes in the fingertips of leprosy patients. J. Neurol. Neurosurg. Psychiatry 54(1991)965-971.
5. BURCHARD, G. -D. and BIERTHER, M. An electron microscopic study of the small cutaneous vessels in lepromatous leprosy. Int. J. Lepr. 53(1985)70-74.
6. EDELBERG, R. and WRIGHT, D. J. Two galvanic-skin response effector organs and their stimulus specificity. Psychophysiology 1(1964)39-47.
7. JOB, C. K. Nerve damage in leprosy. Int. J. Lepr. 57(1989)532-539.
8. KARANTH, S. S., SPRINGALL, D. R., LUCAS, S., LEVY, D., ASHBY, P., LEVENE, M. M. and POLAK, J. M. Changes in nerves and neuropeptides in skin from 100 leprosy patients investigated by immunocytochemistry. J. Pathol. 157(1989) 15-26.
9. Low, P. A. Quantitation of autonomic responses. In: Peripheral Neuropathy, Dyck, P. J., Thomas, P. K., Lambert, F. H. and Bunge, R., eds. Philadelphia: W. B. Saunders, 1984, pp. 1139-1165.
10. Low, P. A. Autonomic nervous system function. J. Clin. Neurophysiol. 10(1993)14-27.
11. Low, P. A., NEUMANN, C, DYCK, P. J., FEALEY, R. D. and TUCK, R. R. Evaluation of skin vasomotor reflexes by using laser Doppler velocimetry. Mayo Clin. Proc. 58(1983)583-592.
12. MASELLI , R. A., JASPAN, J. B., SOLIVEN, B. C, GREEN, A. J., SPIRE, J. -P. and ARNASON, B. G. W. Comparison of sympathetic skin response with quantiatige sudomotor axon reflex test in diabetic neuropathy. Muscle Nerve 12(1989)420-423.
13. RAMU, G. Assessment of the risk of developing leprosy among contacts. Health Cooperation Papers 7(1988) 93-99.
14. RAO. P. S. S., KARATA. B. A., KALIAPERUMAL, V. G. and KARAT, S. Transmission of leprosy within households. Int. J. Lepr. 43(1975)45-54.
15. SHAHANI, B. T., HALPERIN, J. J., BOULU, P. and COHEN, J. Sympathetic skin response: a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J. Neurol. Neurosurg. Psychiatry 47(1984)536-542.
16. SHETTY, V. P., ANTIA, N. H. and JACOBS J. M. The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
17. SHETTY, V. P., MEHTA, L. N. and ANTIA, N. H. Unmyelinated fibres in leprosy neuritis; an ultra-structural study. Bull. Electron Microscop. Soc. India 2(1978)2-5.
18. SHETTY, V. P., MEHTA, L. N., ANTIA, N. H. and IRANI, P. F. Teased fibre study of early nerve lesions in leprosy and contacts, with electrophysiological correlates. J. Neurol. Neurosurg. Psychiatry 40(1977)708-711.
19. SOLIVEN, B. C, MASELLI, R. A., JASPAN, J. B., GREEN, A. J., GRAZIANO, H., PETERSEN, M. and SPIRE, J. P. Sympathetic skin response in diabetic neuropathy. Muscle Nerve 10(1987)711-716.
20. VAN BRAKEL, W. H. and KHAWAS, I. B. Nerve damage in leprosy: an epidemiological and clinical study of 396 patients in west Nepal - Part 1. Lepr. Rev. 65(1994) 204-221.
21. WILDER-SMITH, E., WILDER-SMITH, A., VAN BRAKEL, W. H. and EGGER, M. Vasomotor reflex testing in leprosy patients, healthy contacts and controls: a cross-sectional study in western Nepal. Lepr. Rev. (in press)
1. M.D., D.T.M.&H., Medizinische Poliklinik, Bern, Switzerland.
2. M.D., D.T.M.&H., Neurological Department, Inselspital, CH3010 Bern, Switzerland.
Reprint requests to Dr. Einar Wilder-Smith at above address or fax 41-31 -632-9679.
Received for publication on 17 June 1996; accepted for publication on 17 July 1996.