- Volume 64 , Number 4
- Page: 411–47
Leprosy in Hawaii; the end of an epidemic
Editorial opinions expressed are those of the writers.
During the past 150 years immigrants have introduced leprosy to virtually every major Pacific island settled originally by leprosy-free Polynesians and Micronesians.1-4 Mycobacterium leprae has no known nonhuman reservoir in those areas (no nine-banded armadillos or monkeys). The two different epidemiologic patterns of leprosy revealed in Hawaii during the past 150 years must, therefore, have resulted from the interplay between this infectious agent and a variety of human hosts. The first pattern has been the continuing importation of the agent into Hawaii via infected immigrants. The second pattern has been the transmission of infection among people born in Hawaii, who have endured a large epidemic, especially among native Hawaiians. This epidemic was officially recognized in the 1860s, peaked in the 1890s, gradually decreased over the following three or four generations, and is now approaching extinction.
Infected people who develop only "paucibacillary" disease appear not to transmit the infection toothers. However, if children living in poverty undergo close (household) exposure to someone with untreated "multibacillary"3 disease, about 10% will develop clinically recognizable disease after an incubation period of 1-20 years (usually within 3-5 years). An occasional case may occur among the aged, either after a prolonged incubation period ("late breakdown") or from an infection later in life (a pattern analogous to M. tuberculosis infection). The risk of overt disease appears to be less than 10% among adults who are exposed to untreated "multibacillary" disease in well-to-do environments.3,5-8
Even under the worst conditions, the vast majority of those who are exposed never come down with the disease, thus demonstrating the importance of host resistance against this agent.
Data sources. Since the 1860s the Hawaii Board of Health and its successor the Department of Health (DOH) have maintained active case-finding of leprosy and registration of every new case. In 1948, 1949, and 1950 the late Dr. W. Lloyd Ay-cock, then Professor of Preventive Medicine, Harvard Medical School, visited Hawaii at the invitation of the DOH to examine ethnic and genealogical trends in the leprosy registry and in selected case records. After his death his unpublished working notes were turned over to the author of this paper. Figure 1 is a summary of Dr. Aycock's incidence data covering 1866-1945. Figure 2 and Figure 3 were constructed by the author from the registry, from DOH statistical reports, and from demographic summaries of new cases during the most recent decade.
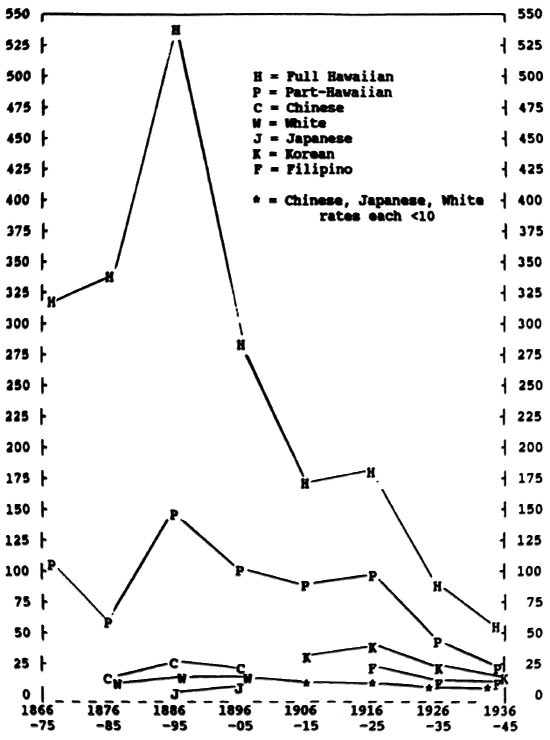
Fig 1. Annual incidence rates of new cases of leprosy per 100,000 by major ethnic group, Hawaii 1886-1945 (pre-sulfone era).
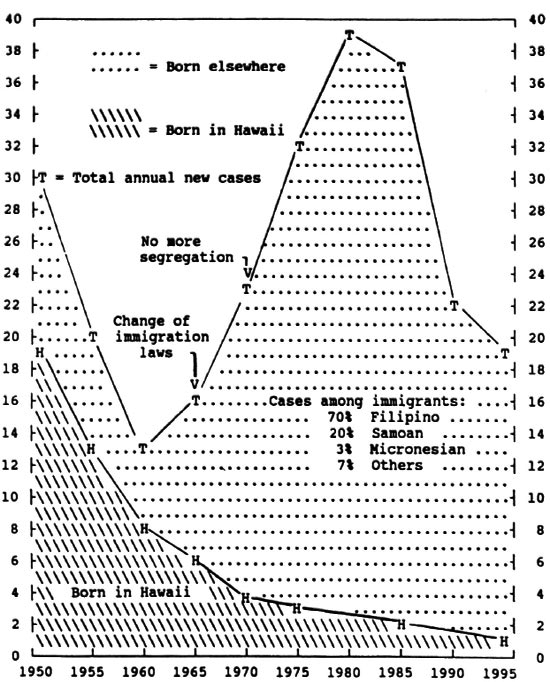
Fig 2. Smoothed annual number of new cases of leprosy in Hawaii per year, by place of birth, 1950-1995.
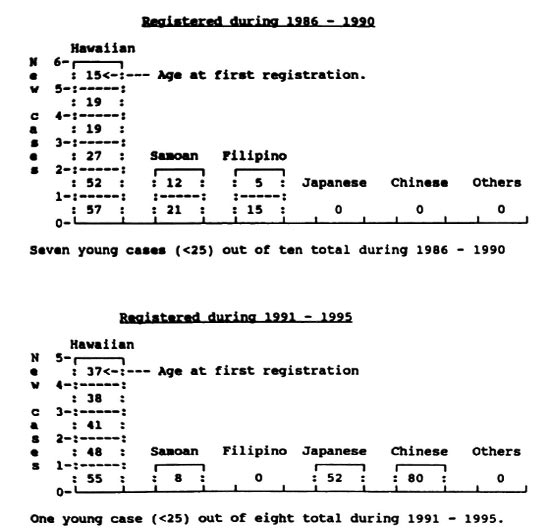
Fig 3. Number of new Hawaii-born cases of leprosy (no history of residence other than Hawaii) by ethnicity and by age at first registration,
Discussion. There is no substantial historical report of leprosy among native Hawaiians before the 1850s, but the subsequent epidemic suggests importation of the bacillus in that era, followed by its wide local transmission. The spread of the disease among Hawaiians became so obvious by the mid-1860s that the DOH initiated mandatory segregation in an attempt to quell the outbreak.
Figure 1 shows the smoothed, average, annual incidence rates per 100,000 people in each major ethnic group in Hawaii during the era when no effective therapy was available, and segregation was the mainstay of public policy. During the first decade of the registry (1866-1875), 1587 cases were found among all ethnic groups. Ninety-nine percent of these first-decade cases were of Hawaiian or part-Hawaiian ancestry. Only 6 Caucasians, 7 Chinese, and 3 "others" were registered in that decade (too few for computing accurate incidence rates).
Case-finding efforts during the first 20 years of the registry were not fully productive, since no treatment was offered and life-long segregation was very unpopular among those who were afflicted. More rigorous case-finding during the 1880s and 1890s produced the peak incidence rates shown in Figure 1 for Hawaiians (over 500 new cases per year per 100,000) and part- Hawaiians (over 140 per year).
By the turn of the century rates of new leprosy cases among Hawaiians had fallen to just above half of their peak, while the rates among part-Hawaiians persisted on an irregular plateau around 100. Early rates for Chinese were around 20 per year, falling steadily thereafter to 6 by 1945. Early Caucasian rates (largely among Portuguese immigrants) were around 16 per year, falling to 2 per year by 1945. The large influx of Japanese to Hawaii began in 1885, bringing only one case of leprosy registered before the turn of the century. Their annual incidence rate rose to 8 in the 1920s, falling gradually thereafter.
Korean and Filipino cases first appeared in about 1910, with the Korean rate peaking at 38 in 1920, and the Filipino rate peaking at 20 in 1920. Rates for both groups fell to 12 by 1945.
During the period from 1900 to 1945 (about two generations), the rates for Hawaiians continued steadily downward to 50 in 1945, while rates among part-Hawaiians moved downward to 22. Although leprosy incidence rates among full and part-Hawaiians were falling, they continued to be higher than other ethnic groups in Hawaii. It should be noted that the total number of full Hawaiians in the population fell from about 30,000 in 1900 to about 12,000 in 1945, while in the same period part-Hawaiians increased from about 9,000 to about 74,000, a product of out-marriage, high fertility, and falling infant mortality. In the same two generations the total population of Hawaii rose from about 150,000 to 490,000.
Figure 2 shows changes from 1950 to the present, with the number of new Hawaii-born cases falling so low (one or two per year) that reliable ethnic incidence rates cannot be computed. The critical variable becomes, therefore, the place of birth, used as proxy for the place of infection (where a person probably was infected while growing up).
Several events of epidemiologic importance took place during the 45-year period shown in Figure 2. The first was the discovery in the 1940s that sulfone drugs are effective against M leprae . Policies were therefore changed so that patients presenting with paucibacillary disease were no longer institutionalized but were treated at home from the outset. This was far less disruptive than the previous policy, which confined such patients for several months to see if they moved toward the ominous multi-bacillary pathway. Treating paucibacillary patients at home also minimized social stigma, resulting in a long step toward cooperation between physicians and patients.
The second event was a change in U.S. immigration laws in 1965, producing a large influx from Southeast and East Asia, especially from The Philippines. The result,shown in Figure 2, was a rapid rise in average numbers (smoothed at 5-year intervals) to 36 new immigrant cases per year in 1980, but falling sharply thereafter. About 70% of cases in this recent immigrant "epidemic" are coming from The Philippines, 20% from Samoa, and 3% from Micronesia, with the remaining 7% from a variety of other places. These immigrant cases arenot producing a second epidemic among people born in Hawaii, whose numbers new cases continue to fall, now down to less than one per year.
The advent of multidrug therapy in the early 1970s led to treating all new cases at home from the beginning, thus ending 105 d years of compulsory isolation. Case management is now handled by selected community physicians in conjunction with the DOH. Rapid bacillary death from early multidrug treatment provides "chemical isolation," thus making physical isolation irrelevant.
Figure 3 shows in detail the onset of leprosy in the 18 Hawaii-born patients who have no history of living elsewhere and who were registered as new cases during 1986-1995. The end of the epidemic among Hawaiians/part-Hawaiians is clearly visible. It is encouraging to note that only three young Hawaiian/part-Hawaiian cases (under age 25 and therefore probably due to recent transmission) were registered in the 1986-1990 period (see top of Fig. 3). The last such case was born in 1972 and was diagnosed in 1987-no more young Hawaiian cases have been seen since then.
In the ten-year period covered by Figure 3, there are three young Hawaii-born Samoan cases and two young Hawaii-born Filipino cases, probably indicating some recent transmission from immigrant relatives. The two recent elderly Hawaii-born Chinese and Japanese cases very likely represent "late breakdown" from infection acquired many years ago, when the disease was still quite prevalent in Hawaii's population.
It would seem that the epidemiologic situation in Hawaii has changed in recent generations. The numbers of secondary multi-bacillary cases being produced by transmission from new (or relapsed) multibacillary cases appear to have become too small to perpetuate the epidemic in Hawaii, even though re-introduction of the bacillus continues. The epidemic is therefore declining, probably due to increasing human resistance to the bacillus rather than due to a lack of exposure, which continues.
During the last half of the 1800s the Hawaiians underwent the stresses of displacement from the land, loss of subsistence agriculture, and an overwhelming move to a money economy. These changes were clearly accompanied by excess mortality and morbidity from many new diseases, often accompanied by poor nutrition. Accommodation to these changes in recent generations has included improvement of nutrition through government-sponsored programs for the poorer segments of the population. This may have improved general immunologic competence against leprosy, even though the bacillus continues to be imported. It also has been suggested that the changing genetics of Hawaii may have been a help, due to an admixture in part-Hawaiians of genes from Asians and Europeans whose ancestors have survived centuries of severe social stigma against leprosy. When social stigma operates against a young person, it selects against reproduction, which would lead to selection in favor of leprosy resistance, thus passing that resistance to out-marrying part-Hawaiians.
The genetic hypothesis is undermined, however, by a leprosy epidemic that disappeared spontaneously among the 180-250 Hawaiians living on the isolated island of Niihau, where the socioeconomic stresses have been greatly minimized by the owners of the island. These islanders are preponderantly of full Hawaiian ancestry, and the Hawaiian language continues to be the primary tongue. The first leprosy cases were discovered in the 1870s, and the last case in 1939-34 cases in all. Since the genetics have not changed in this little inbred population, the end of this epidemic must have been due to some other factor.9
Another leprosy epidemic has been described in a central Pacific "virgin soil" island population (Nauru). This involved a sudden rise in the number of cases in the 1920s after introduction of a single multi-bacillary case from another location.1,2 Laborers returning home to Micronesia after working in Nauru have resulted in three persistent outbreaks of leprosy in Micronesia. These foci are in subpopulations undergoing socioeconomic stress while moving to a money economy and losing traditional diets. Active case-finding and treatment have been tried sporadically in these three foci over the past 30-40 years, but without long-term success.
The importance of the genetic hypothesis is further undermined by the great scarcity of secondary leprosy cases found in England, Holland, Israel, and Hawaii among locally bom children exposed to their multi-bacillary immigrant family members.
Summary. Two different patterns of leprosy have occurred in Hawaii. First is the continuing influx of infected people among immigrants from several leprosy-endemic areas. The number of new cases among their descendents has tended to abate within one generation after arrival in Hawaii. The most recent example of this has occurred in Asians arriving since immigration laws were liberalized in 1965, followed by a rise of imported leprosy, peaking in 1970-1980, then falling. The second pattern was an explosive epidemic among native Hawaiians, rising to its peak in the 1890s, then slowly subsiding, and now approaching zero. It appears that official disease-control efforts (physical isolation and/or early multidrug treatment) cannot fully explain the ending of the epidemic in Hawaii, in spite of the continuing importation of M. leprae. It is suggested that the influence of changing socioeconomic factors (nutrition, crowding, genetics) has been of importance, particularly among the Hawaiians, who have undergone profound foreign influence (both positive and negative) during the 150-year history of this epidemic.
The late Dr. Ma Haide was responsible for leprosy control in China, where leprosy is also fading away, but not simultaneously in all subpopulations. He summarized his view by saying, "Leprosy lingers longest among the poorest" (personal communication). This statement appears to hold true for Hawaii, also.
- Robert M. Worth, M.D., Ph.D.
Senior Investigator
Pacific Health Research Institute
846 South Hotel Street, Suite 303
Honolulu, HI 96813
Acknowledgment. Drafts of this paper were reviewed by Drs. O. A. Bushnell, Richard Frankel, and Alfred Morris who have offered helpful comments. Mr. Michael Moriyama of the Hawaii Department of Health has provided abstracts of all new cases registered in Hawaii during1986-1995, from which the author produced Figure 3. Any residual errors are attributable to the author.
1. Morgan, F. G. Report on the investigation of a number of eases of leprosy in Nauru, Central Pacific. Commonwealth of Australia, Department of Health, l992. Ser. Publ. No.25.
2. Wade, H. W. and Ledowsky, V. The leprosy epidemic at Nauru. Int. J. Lepr. 20(1952)1-19.
3. WHO Expert Committee on Leprosy. Sixth report. Geneva: World Health Organization, 1982, pp. 11-15. Tech. Rep. Ser. 768.
4. WHO Regional Office for the Western Pacific. Regional leprosy situation: country reports. Manila: World Health Organization, 1992.
5. WHO Action Programme for the Elimination of Leprosy. Geneva: World Health Organization, 1995, pp. 15-26.
6. Worth, R. M. and Hirschy, I. B. A test for the infectivity of tuberculoid leprosy. Hawaii Med. J. 24(1964)116-119.
7. Worth, R. M. Is it safe to treat the lepromatous patient at home? Int. J. Lepr. 36(1986)2296-2302.
8. Worth, R. M. and Wong, K. O. Further notes on the incidence of leprosy in Hong Kong children living with a lepromatous parent. Int. J. Lepr. 39(1971)744-748.