- Volume 60 , Number 4
- Page: 556–61
Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice
ABSTRACT
The bactericidal activities against Mycobacterium leprae of single or multiple doses of various combinations of new antileprosy drugs [minocycline (MINO), clarithromycin (CLARI), ofloxacin (OFLO), and sparfloxacin (SPFX)] and/or rifampin (RMP) were titrated in immunocompetent mice by the proportional bactericidal method. Drugs were administered by gavage at the following dosages (mg/kg) per dose: RMP 10, MINO 25, CLARI 100, OFLO 150, and SPFX 50. All 15 regimens exerted significant bactericidal activities, at least 96% of viables were killed. The activity of a single dose MINO + CLARI was only slightly inferior to that of RMP, and the activities of a single dose OFLO/SPFX + MINO + CLARI were similar to that of RMP. This suggests that either MINO + CLARI or OFLO/SPFX + MINO + CLARI may be administered once monthly together with RMP 600 mg for the treatment of multibacillary (MB) leprosy, and monthly administration of MINO + CLARI or OFLO/ SPFX + MINO + CLARI may also be employed for the treatment of RMP-resistant MB leprosy. Because the killing effects of multiple doses of the combinations were so powerful, comparison of the bactericidal activities of these regimens was beyond the sensitivity of the immunocompetent mouse model, and are being tested in the nude mouse model. Although SPFX is more active against M. leprae than OFLO on a weight-to-weight basis, when both drugs were administered in mice at dosages equivalent to clinically tolerated dosages in humans, SPFX did not show more superiority than OFLO, and its real advantage over OFLO in the treatment of leprosy remains unclear.RÉSUMÉ
L'activité bactéricide vis-à-vis de Myeobacterium leprae d'une dose unique ou de doses multiples de diverses combinaisons de nouveaux médicaments antilépreux [minocycline (MINO), clarithromycinc (CLARI), olloxacinc (OFLO), et la sparfloxacine (SPFX)] et/ ou la rifampicine (RMP) a été titrée chez des souris immunocompétentes par la méthode bactéricide proportionnelle. Les médicaments ont été utilisés par gavage aux dosages suivants (mg/kg): RM P 10, MIN O 25, CLARI 100, OFLO 150, et SPFX 50. Les 15 régimes ont montré une activité bactéricide significative, au moins 96% des bacilles viables étant tués. L'activité d'une dose unique de MINO + CLARI était seulement légèrement plus faible que celle de RMP, et l'activité d'une dose unique de OFLO/SPFX + MINO + CLARI était similaire à celle de RMP. Ceci suggère que MINO + CLARI ou OFLO/SPFX + MINO + CLARI peut être administré une fois par mois avec RMP 600 mg pour le traitement de la lèpre multibacillairc (MB), et une administration mensuelle de MINO + CLARI ou OFLO/SPFX + MINO + CLARI peu également être utilisée pour le traitement de la lèpre MB résistant à la RMP. Comme l'effet bactéricide des doses multiples des combinaisons était si puissant, la comparaison de l'activité bactéricide de ces régimes n'était pas possible du fait de la sensibilité du modèle de la souris immunocompétente; cette activité est présentement comparée à l'aide du modèle de la souris nue. Bien que SPFX soit plus actif vis-à-vis de M. leprae que OFLO à doses équivalentes à celles cliniquement tolérées chez l'homme, SPFX ne s'est pas montré supérieur à OFLO, et son avantage reel stir OFL O dans le traitemcnt de la lepre reste incertain.RESUMEN
Utilizando el método bactericida proporcional, se titularon las actividades bactericidas contra el Mycobacterium leprae de varias combinaciones de nuevas drogas (minociclina, MINO; claritromicina, CLARI; ofloxacina, OFLO; y esparfloxacina, SPFX), y de rifampina (RMP), administradas como dosis únicas o múltiples en ratones inmunocompetentes. Las drogas se administraron por via oral a las siguientes dosis (mg/ kg): RMP 10, MINO 25, CLARI 100, OFLO 150, y SPFX 50. Los 15 esquemas probados ejercieron significantes actividades bactericidas y al menos el 96% de los bacilos resultaron muertos. La actividad de una sola dosis de MINO + CLARI Tue solo ligeramente inferior a la de la RMP. La lepra multibacilar (MB) puede ser tratada con una sola dosis de OFLO/SPFX + MINO + CLARI, una vez al mes junto con 600 mg de RMP. La administración mensual de MIN O + CLARI o de OFLO/SPFX + MINO + CLARI, puede emplearse también para el tratamiento de la lepra MB resistente a la RMP. Debido al intenso efecto bactericida de las diversas combinaciones de drogas, la comparación de las actividades bactericidas de estos esquemas de tratamiento estuvo más allá de la sensibilidad del modelo del ratón inmunocompetente, y ahora ésto se está estudiando en el ratón desnudo. Aunqu la SPFX es más activa contra el M. leprae que la OFLO, cuando ambas drogas se administraron en el ratón en dosis equivalentes a las toleradas en el humano, la SPFX no resultó ser superior a la OFLO. La ventaja real de la SPFX sobre la OFLO, en el tratamiento de la lepra, no es clara.Rifampin (RMP) displays very rapid and powerful bactericidal activity against Mycobacterium leprae in lepromatous leprosy (1, 16, 23, 24). The bacilli lose their infectivity to immunocompetent mice after patients receive treatments with single doses of 600 mg, 900 mg, and 1200 mg RMP (16). As in human leprosy, significant bactericidal activity against M. leprae, as titrated by the proportional bactericidal method, of a single dose of RMP 10 mg/kg in immunocompetent mice has been well documented (8, 13,15)with very few exceptions(18). Therefore, monthly supervised administration of RMP 600 mg is the backbone of the multidrug therapy (MDT) regimens for both paucibacillary (PB) and multibacillary (MB) leprosy recommended by a WHO Study Group (27). The major objective of incorporating two other drugs, dapsone (DDS) and clofazimine (CLO), in the regimen for MB leprosy (27) is to prevent the selection of RMP-resistant mutants. However, close to 30% of patients in Karigiri, India, one of the best leprosy control programs in the world and where CLO is well accepted by the patients because of their dark skin, did not take their prescribed DDS and CLO properly (3), suggesting that simply because of noncompliance, it is still possible to develop RMP resistance in a program where MDT is implemented. The risk may be significantly reduced ifa fully supervised MDT regimen is developed such that all components are administered once monthly under supervision. The basic requirements for such a component should be: a) its single dose displays a certain degree of bactericidal activity against M. leprae, and b) the dosage is well tolerated by the patients.
Recently, three different classes of antimicrobials with powerful bactericidal activities against M. leprae have been identified, namely, pefloxacin (PEFLO) (10, 11, 17, 19), ofloxacin (OFLO) (9, 10, 19) and sparfloxacin (SPFX)(5, 26) (fluoroquinolones); clarithromycin (CLARI) (4, 12, 15) (macrolide); and minocycline (MINO) (6, 7, 15) (tetracycline). Because a) three doses of PEFLO 300 mg/ kg once every 4 weeks in mice did not show any bactericidal activity (19), b) single-dose PEFLO or OFLO 800 mg only displayed modest bactericidal activity in humans (10) and c) multiplication of M. leprae was observed in 4 of the 5 strains of mice which were fed with MINO-containing mouse diet at a concentration of 0.04% (ca. 80 mg/kg/ day) 1 day monthly for 6 or 9 months (7), the bactericidal activities of single doses of the new drugs alone are relatively weak. However, additive effects were observed in mice with the combination of CLARI and MINO, and of CLARI, MINO and RMP(15); it is possible that single doses of the combinations of these new drugs may display significant bactericidal activity against M. leprae. To evaluate the possibilities, we have measured the bactericidal activities of single or multiple doses of various combinations of these new drugs, with or without RMP, in mice with the proportional bactericidal method (2).
MATERIALS AND METHODS
M. leprae. Strain 90002 was isolated from a previously untreated lepromatous patient, and passaged through nude mouse inoculation. The strain was fully susceptible to both DDS and RMP. An inoculum containing 1 × 104 M. leprae per 0.03 ml was prepared (20), and serial 10-fold dilutions were made, so that the final dilution contained 1 × 100 organisms per 0.03 ml.
Mice. Six-hundred-twenty (620) female immunocompetent Swiss mice were purchased from a local supplier.
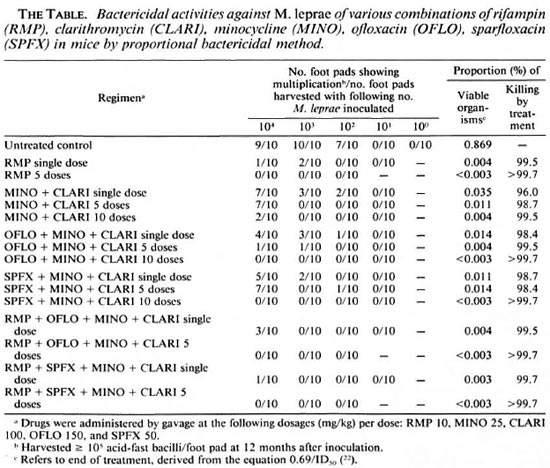
Mouse inoculation. Mice were divided into five groups. Each hind foot pad of the mice in the first three groups, with 160 mice each, was inoculated with 0.03 ml of suspension containing, respectively, 1 × 104, 1 × 103, or 1 × 102 organisms. Mice in the fourth and fifth groups, respectively, 130 and 10 mice, were inoculated with 1 × 101 or 1 × 100 organisms. The inoculated mice were reallocated into 16 groups, 1 control and 15 treated groups, as shown in The Table.
Treatments. Beginning from day 3 after inoculation, the various regimens were administered by gavage once daily, ranging from single doses to 10 doses as shown in The Table. The control mice were not treated. Drugs were suspended in 0.05% agardistilled water at the following dosages per dose: RMP 10 mg/kg, MINO 25 mg/kg, CLARI 100 mg/kg, OFLO 150 mg/kg, and SPFX 50 mg/kg. The dosages were chosen to provide the areas under concentration curves (AUC) in mice comparable to those obtained in man with clinically tolerated dosages, i.e., RMP 600 mg, MINO 100 mg, CLARI 1000 mg, OFLO 600 mg, and SPFX 200 mg daily.
Harvests. The soft tissues of the inoculated foot pads were harvested (20) individually at 12 months after inoculation; in other words, the mice were held without treatment for at least 11.5 months, a period of time theoretically sufficient for one surviving organism to multiply to a level of > 105 per foot pad, the criterion of multiplication of M. leprae.
Statistical analysis. The proportion of viable organisms remaining at the end of treatment and the significance of differences between groups were calculated by the Spearman and Karbcr method (22).
RESULTS
The results of the harvests, the proportion of the viable organisms at the end of treatment, and the proportion of the viable organisms killed by the treatments are presented in The Table.
The results of multiplication of M. leprae in the untreated control mice indicate that the proportion of viable organisms in the inoculum was 0.869%. All 15 regimens exerted very significant bactericidal effects compared with the control group (p < 0.01). As positive controls, single and 5 doses of RMP killed, respectively, 99.5% and > 99.7% of the viable bacilli.
Among the single-dose regimens, the bactericidal activity of MINO + CLARI, 96.0%, was significantly smaller than that of RMP (p < 0.01), but the activity of OFLO + MINO + CLARI or SPFX + MINO + CLARI did not differ significantly from that of RMP (p > 0.05). The activity of OFLO + MINO + CLARI was virtually the same as that of SPFX + MINO + CLARI. Although both were greater than that of MINO + CLARI, the differences did not attain statistical significance (p > 0.05). The activity of RMP + OFLO + MINO + CLARI or RMP+ SPFX + MINO + CLARI was very similar to that of single-dose RMP (p > 0.05).
The bactericidal activity of multiple (5 or 10) doses of any of the combinations was very powerful; except for 5 doses of MINO + CLARI and SPFX + MINO + CLARI, all other regimens killed at least 99% of viable organisms. No multiplication of M. leprae was observed in mice after 10 doses of treatment with OFLO + MINO + CLARI or SPFX + MINO + CLARI, or any regimens containing 5 doses of RMP (either alone or in combination). A comparison of the bactericidal activities between 5 doses of OFLO + MINO + CLARI and three other regimens containing 5 doses of RMP (either alone or in combination) is difficult because multiplication of M. leprae was observed among very few mice in the former group and no multiplication was observed in the latter groups. However, the activities of all four regimens were significantly greater than 5 doses of MINO + CLARI or SPFX + MINO + CLARI (p < 0.01). The bactericidal activity of 10 doses of MINO + CLARI was exactly the same as that of 5 doses of OFLO + MINO + CLARI, significantly greater than that of 5 doses of MINO + CLARI (p < 0.01). The latter was also greater than that of a single dose of MINO + CLARI but did not attain statistical significance (p > 0.05).
DISCUSSION
It is encouraging to observe that singledose MINO + CLARI exerted a significant bactericidal effect against M. leprae in mice, although its activity was slightly inferior to that of single-dose RMP. The bactericidal activity of a single dose of OFLO + SPFX + MINO + CLARI was similar to that of single-dose RMP, and neither a synergistic nor an antagonistic effect was detected between single-dose RMP and the combinations of OFLO/SPFX + MINO + CLARI. Because the dosages of these new antileprosy drugs in mouse experiments were comparable to clinically tolerated dosages in humans, i.e., MINO 100 mg, CLARI 1000 mg, OFLO 600 mg, or SPFX 200 mg daily, this suggests that either MINO + CLARI or OFLO/SPFX + MINO + CLARI (with the above-mentioned dosages) may be administered once monthly together with RMP 600 mg for the treatment of MB leprosy. In addition, monthly administration of MINO + CLARI or OFLO/SPFX + MINO + CLARI may also be employed for the treatment of RMP-resistant MB leprosy. Although the bactericidal activity of daily DDS + CLO has not been titrated in mice by the proportional bactericidal method, the results of the kinetic method indicated that no additive effect was detected by the combination of daily DDS + CLO (21). Because 30 days of daily treatment with 0.01% DDS in the mouse diet killed 72% (2) to 87% (13) of viable bacilli, and 0.003% CLO in mouse diet killed 96% (2) of viable bacilli, it is reasonable to assume that the bactericidal activity of a single dose of MINO + CLARI or OFLO/SPFX + MINO + CLARI should be the same as that of a 1-month treatment with daily DDS + CLO and, therefore, the fully supervised monthly administered regimens of the new drugs in combination with RMP will be as active as the standard MDT regimen for MB leprosy. A clinical trial to compare the bactericidal activities of single doses of MINO + CLARI or OFLO + MINO + CLARI versus 1 month of daily DDS + CLO is being conducted in previously untreated lepromatous leprosy.
To develop multidrug regimen(s) in which the total duration of treatment could be significantly shortened to less than 2 years or less than 6 months, the most feasible approach is to combine various powerful bactericidal drugs and to continue the treatment until a great majority of viable organisms, especially all drug-resistant mutants, are killed (14). The bactericidal activities of multiple (5 or 10) doses of various drug combinations were measured in the current experiment. Except for 5 doses of MINO + CLARI or SPFX + MINO + CLARI, the killing effects of multiple doses of various combinations, even without RMP, were so powerful that at least 99% of the viable bacilli were killed, or no more viables were detected (i.e., > 99.7% of killing). Therefore, a comparison of the bactericidal activities of these regimens was beyond the sensitivity of the immunocompetent mouse model. For this very reason, the initial killing of the viables by the treatment of multiple doses of various combinations is being evaluated in the nude mouse model (14).
Although SPFX is more active against M. leprae than OFLO on a weight-to-weight basis (5, 26), the greater activity of SPFX is counterbalanced by its lower clinically tolerated dosage (200 mg to 400 mg daily vs OFLO 400 mg to 800 mg daily). In terms of AUC, 200 mg of SPFX in humans is equivalent to 50 mg/kg in mice (unpublished data), and OFLO 600 mg in humans is equivalent to 150 mg/kg in mice (25). Therefore, in the current mouse experiment, SPFX and OFLO were administered, respectively, at 50 mg/kg/dose and 150 mg/ kg/dose. The bactericidal activities of regimens containing single and 10 doses of SPFX were very similar to those containing the same doses of OFLO, but the activity of 5 doses of SPFX + MINO + CLARI was significantly less than that of OFLO + MINO + CLARI. Therefore, the real advantage of SPFX over OFLO in the treatment of leprosy remains unclear.
REFERENCES
1. COLLABORATIVE EFFORT OF THE U.S. LEPROSY PANEL (U.S.-JAPAN COOPERATIVE MEDICAL SCIENCE PROGRAM) and THE LEONARD WOO D MEMORIAL. Rifampin therapy of Lepromatous leprosy. Am. J. Trop. Med. Hyg. 24(1975)475-484.
2. COLSTON, M. J., HILSON, G. R. F. and BANERJEE, D. K. The "proportional bactericidal test": a method for assessing bactericidal therapy on the growth of Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 35(1991)992-994.
3. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN, M. Clofazimine and dapsone compliance in leprosy. Lepr. Rev. 59(1988)205-223.
4. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
5. FRANZBLAU. S. G. and WHITE, K. E. Comparative in vitro activities of 20 fluoroquinolones against Mycobacterium leprae. Antimicrob. Agents Chemother. 34(1990)29-231.
6. GELBER, R. H. Activity of minocycline in Mycobacterium /cprac-infected mice. J. Infect. Dis. 156(1987)236-239.
7. GELBER, R. H., SIU, P., TSANG, M., ALLEY, P. and MURRAY. L. P. Effect of low-level and intermittent minocycline therapy on the growth of Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 35(1991)1992-1994.
8. GROSSET, J.-H. and GUELPA-LAURAS, C.-C. Activity of rifampin in infections of normal mice with Mycobacterium leprae. Int. J. Lepr. 55(1987)847-851.
9. GROSSET, J.-H., GUELPA-LAURAS, C.-C, PERANI, E. G. and BEOLETTO, C. Activity of ofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 56(1988)259-264.
10. GROSSET, J.-H., JI, B., GUELPA-LAURAS, C.-C, PERANI, E. G. and N'DELI, L. N. Clinical trial of pefloxacin and ofloxacin in the treatment of Lepromatous leprosy. Int. J. Lepr. 58(1990)282-295.
11. GUELPA-LAURAS, C.-C, PERANI, E. G., GIROIR, A. M. and GROSSET, J.-H. Activities of pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
12. JAMET, P., JI, B., BOBIN, P. and GROSSET, J.-H. Powerful bactericidal activities of clarithromycin and/or minocycline against M. lepraein man. (Abstract 970) 31st ICAAC, Sept. 28-Oct. 2, 1991, Chicago, Illinois, U.S.A.
13. Ji, B., CHEN, J., Lu, X., WANG, S., NI, G., HOU, Y., ZHOU, D. and TANG, Q. Antimycobacterial activities of two newer ansamycins, R-76-1 and DL 473. Int. J. Lepr. 54(1986)563-577.
14. Ji, B. and GROSSET, J.-H. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61(1990)313-329.
15. Ji, B.. PERANI, E. G. and GROSSET. J.-H. Effectiveness of clarithromycin and minocycline alone or in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
16. LEVY. L., SHEPARD, C. C. and FASAL. P. The bactericidal effect of rifampicin on M. leprae in man: a) single doses of 600, 900 and 1200 mg; and b) daily doses of 300 mg. Int. J. Lepr. 44(1976)183-187.
17. N'DELI. L., GUELPA-LAURAS. C.-C. PERANI, E. G. and GROSSET, J.-H. Effectiveness of pefloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)12-18.
18. PATTYN, S. R. A comparison of the bactericidal activity of a scries of rifampicins against Mycobacterium leprae. Arzneimittelforsch. 32(1982)15-17.
19. PATTYN, S. R. Activity of ofloxacin and pefloxacin against Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 31(1987) 671-672.
20. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into footpads of mice. J. Exp. Med. 112(1960)445-454.
21. SHEPARD, C. C. Combination involving dapsone, rifampin, clofazimine, and ethionamide in the treatment of M. leprae infections in mice. Int. J. Lepr. 44(1976) 135-139.
22. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1982)96-101.
23. SHEPARD, C. C, LEVY, L. and FASAL. P. Rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
24. SHEPARD, C. C, LEVY, L. and FASAL, P. Further experience with the rapid bactericidal clfect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120- 1124.
25. TRUFFOT-PERNOT, C, JI, B. and GROSSET, J.-H. Activities of pefloxacin and ofloxacin against mycobacteria: in vitro and mouse experiments. Tubercle 72(1991)57-64.
26. TSUTSUMI, S. and GIDOH, M. Studies on the development of novel chemotherapeutics using nude mice with special reference to a new quinolone carboxylic acid, AT-4140. Jpn. J. Lepr. 58(1989)250-257.
27. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
1. M.D.; Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière. 91 Blvd. de l'Hôpital. 75634 Paris 13. France.
2. Technical Officer; Bactériologie et Virologie, Faculté de Médecine Pitié-Salpêtrière. 91 Blvd. de l'Hôpital. 75634 Paris 13. France.
Received for publication on 30 April 1992.
Accepted for publication on 11 June 1992.