- Volume 60 , Number 4
- Page: 562–9
Suppressive effect of circulating immune complexes f rom leprosy patients on the lymphocyte proliferation induced by M. leprae antigens in healthy responders
ABSTRACT
The effect of circulating immune complexes, isolated in the form of polyethylene glycol (PEG) precipitates f rom leprosy patients, on lymphocyte proliferation was studied. The results obtained showed that PEG precipitates obtained f rom the borderline lepromatous/Iepromatous (BL/LL) types of leprosy patients and those undergoing erythema nodosum leprosum (ENL) had significant suppressive effects on the lymphocyte proliferation induced by Mycobacterium leprae antigens in healthy responders. The percent decreases in the mean values of Δcpm in the presence of PEG precipitates f rom the BL/LL and ENL groups were found to be 46.8 ± 22.4 and 65.0 ± 24.3, respectively. However, no significant suppressive effects (except for ENL PEG precipitates) of these PEG precipitates were observed on the lymphocyte proliferation induced by tuberculin (PPD). Further, PEG precipitates alone (in the absence of M. leprae antigen) f rom the BL/LL and ENL groups were found to have no effect on the lymphocyte proliferation.RÉSUMÉ
On a étudié l'effet, sur la prolifération lymphocytaire, des complexes immuns circulants, isolés de patients lépreux sous la forme de précipités de Polyethylene glycol (PEG). Les résultats ont montré que les précipités de PEG obtenus à partir de patients atteints de lèpre borderline lépromateuse ou lépromateuse (BL/ LL) et ceux présentant un éry thème noueux lépreux (ENL) avaient des effets suppresseurs significatifs sur la prolifération lymphocytaire induite par des antigènes de Mycobacterium leprae chez des personnes en bonne santé. La diminution des valeurs moyennes de Δcpm en présence de précipités de PEG provenant des groupes de patients BL/LL et ENL était respectivement de46.8 ± 22.4%etde65.0 ± 24.3%.Cependant,aucun effet suppresseur significatif (à l'exception des précipités de PEG provenant des patients ENL) de ces précipités de PEG n'était observé vis-à-vis de la prolifération lymphocytaire induite par la tuberculinc (PPD). De plus, on n'a pas trouvé d'effet des précipités de PEG seuls (en l'absence d'antigène de M. leprae) provenant des patients BL/LL et ENL sur la prolifération lymphocytaire.RESUMEN
Se estudió el efecto de los complejos inmunes circulantes aislados por precipitación con polietilen glicol (PEG) de los sueros de pacientes con lepra, sobre la proliferación de linfocitos de individuos respondedores sanos. Los resultados obtenidos mostraron que los precipitados PEG obtenidos de pacientes con lepra lepromatosa/lepromatosa subpolar (LL/BL) y de aquellos pacientes con eritema nodoso leproso (ENL), tuvieron significantes efectos supresivos de la proliferación de linfocitos inducida por los antigenos del Mycobacterium leprae. Los porcentajes de disminución en los valores medios de las CPM en presencia de precipitados PEG de los grupos BL/LL y ENL. fueron 46.8 ± 22 y 65.0 ± 24.3, respectivamente. Sin embargo, los precipitados PEG de los pacientes LL/BL no tuvieron efectos supresivos significantes sobre la proliferación de linfocitos inducida por la tuberculina (PPD). Los precipitados PEG solos de los grupos BL/LL y ENL, en ausencia de antígenos del M. leprae. no tuvieron ningún efecto sobre la proliferación de linfocitos.Elevated levels of circulating immune complexes (CICs) have been demonstrated in the sera of leprosy patients, particularly in patients with borderline lepromatous (BL)/lepromatous (LL) types of leprosy and those with erythema nodosum leprosum (ENL) (1, 4, 28, 29). Further, CICs from BL/LL and ENL patients have been shown to have efficient complement-activating ability (19.28)
Patients with BL/LL types of leprosy and those suffering ENL already have been shown to have diminished cell-mediated immune (CMI) response against Mycobacterium leprae (26, 27). The mechanism of the defective CMI response in patients with LL leprosy is still unclear. Studies have, however, shown that the cellular mechanisms may be responsible for this defect.- Both macrophages (21) and T cells (9) from LL patients have been shown to have suppressor activity. Further, Nath, et al. (13) have shown that some factors from the monocytes of LL patients may induce a suppressive effect.
Godal, et al. (5) suggested that unresponsiveness in LL patients is due to the lack of circulating T cells reactive to M. leprae. Sometimes this M. leprae-specific T-cell unresponsiveness can be corrected by the addition of exogenous interleukin-2 (IL-2) (6, 7). However, the M. leprae-specific response of T cells cannot be restored in all LL patients(14). On the contrary, Mohagheghpour, et al. (11) explained this unresponsiveness as the lack of expression of IL-2 receptors on the lymphocytes of LL patients. Although there have been some studies indicating that blocking antibodies (l5), lymphocytotoxic antibodies (23), and immune complexes (ICs) (1) might play some role in immunosuppression in leprosy, the literature is very scanty and needs further evaluation. Whether humoral components (such as CICs) play any significant role in the suppression of M. leprae-induced lymphocyte proliferation has, therefore, been studied by us. CICs were isolated from the sera of leprosy patients and their effect on in vitro lymphocyte proliferation induced by M. leprae antigens (Ags) was studied.
MATERIALS AND METHODS
Cell-free extract (CFE). The cell-free extract (CFE) of armadillo-derived M. leprae (kindly supplied by Dr. R. J. W. Rees, National Institute for Medical Research, London, from the IMMLEP Bank) was used as specific antigen for the lymphocyte transformation test (LTT).
Purified protein derivative (PPD). PPD of bovine tuberculin, neutralized and freezedried (Ministry of Agriculture, Fisheries and Food, Central Veterinary Laboratory, Way Bridge, Surrey, U.K.) was a gift of LEPRA U.K.
Sera for isolation of CICs. Venous blood samples (10 ml each) were collected from the patients in the outpatient department of the Central JALMA Institute for Leprosy, Agra, India. The patients were classified on the clinical criteria of Ridley and Jopling(18): 4 tuberculoid (TT)/borderline tuberculoid (BT), 5 BL/LL, and 4 ENL subjects. Blood samples from four healthy laboratory volunteers were used as controls.
Isolation of CICs by PEG 6000. CICs were precipitated by 2.5% polyethylene glycol (PEG) 6000 (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) treatment of sera by a previously described method (28).
Source of sensitized mononuclear cells. For lymphocyte proliferation, mononuclear cells (MNCs) were isolated from the peripheral blood of healthy personnel working at our institute who have been in constant exposure to leprosy patients for many years and were known to mount a strong CMI response as determined by leukocyte migration inhibition and lymphocyte proliferation to M. leprae antigens and by in vivo lepromin testing.
Lymphocyte transformation test (LTT). MNCs from healthy responders were isolated by centrifugation on a Ficoll-Hypaque density gradient (2) and cultured as described previously (3). In brief, 2 x 105 cells were cultured in triplicate in flat-bottom microtiter plates (Nunc, Denmark) in 200 µl of RPMI 1640 containing 10% pooled AB+ serum which was supplemented with 2 mM L-glutamine and antibiotics (100 IU/ ml of penicillin and 100 µg/ml streptomycin). The MNCs were stimulated with the optimal antigen doses of 5 µg/well of CFE and 1 µg/well of PPD, and were cultured for 5 days at 37ºC in 5% C02 in humidified air. The cultures were pulsed with 1.0 µCi of tritiated methyl thymidine (specific activity 2.0 Ci/mmol; 3H-TdR, Radiochemicals, Inc., Amersham, U.K.) for 18 hr before harvest. The cells were harvested by a cell harvester (Ilacon Harvester, U.K.) and 3H-thymidine incorporation of the culture was measured in a liquid scintillation counter (LKB Wallac 1209 Rack Beta, Finland).
The results were expressed as mean counts per minute (cpm) of triplicates. The percent decrease in the mean cpm in the presence of ICs was calculated using the following formula:

Agents used in LTT. CFE 10 µl (5 µg); PPD 10 µl (1 µg);NHS PEG precipitates 10 µl with or without CFE/PPD; TT/BT PEG precipitates 10 /til with or without CFE/PPD; BL/LL PEG precipitates 10 µl with or without CFE/PPD; ENL PEG precipitates 10 /d with or without CFE/PPD were used in the LLT.
Statistical analysis. Group means (of percent decrease in Δcpm) were compared by Student's / test.
RESULTS
Effect of PEG precipitates on the LTT. As can be seen in Figure 1, the PEG precipitates isolated from the sera of NHS and BL/LL groups at various concentrations (neat, 1:2, 1:4) were found to have no significant dose-dependent effect (proliferative or suppressive) on the LTT. In addition to this, 10 µl of (neat) PEG precipitates from TT/BT, BL/LL, and ENL groups were also found to have no significant effect on the LTT. The cpm obtained were similar to those of PEG precipitates from normal controls and RPMI 1640 media alone (Fig. 2).
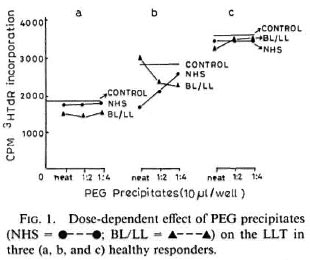
Fig. 1. Dose-dependent effect of PEG precipitates (NHS-●---●; BL/LL = ▲---▲) on the LLT in three (a, b, and c) healthy responders.
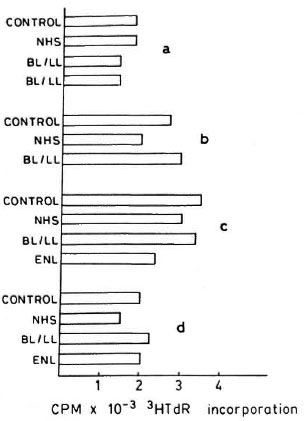
Fig. 2. Effect of PEG precipitates obtained from serum of normal healthy volunteers (NHS), borderline lepromatous (BL)/lepromatous leprosy (LL), and erythema nodosum leprosum (ENL) patients on lymphocyte proliferation of healthy responders (a, b, c, and d); control = RPMI 1640; horizontal bars = mean cpm × 10-3 3H-TdR uptake of triplicate cultures.
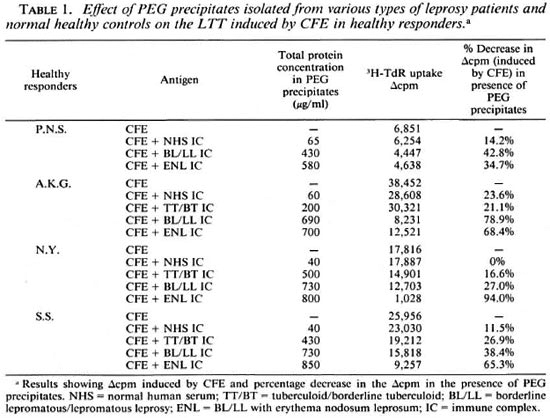
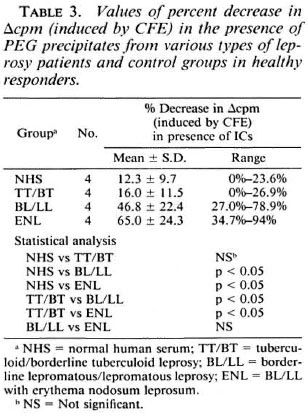
Effect of PEG precipitates on LTT induced by CFE. PEG precipitates from BL/ LL and ENL groups were found to exert a statistically significant suppressive effect on the LTT induced by CFE. PEG precipitates obtained from TT/BT and normal control groups, however, failed to show any such effect. The percent decreases in the mean values of Δcpm obtained were: NHS, 12.32 ±9.7; TT/BT, 15.98 ± 11.51; BL/LL, 46.77 ± 22.42 and ENL, 65.0 ± 24.28. The difference in the mean values of percent decrease between NHS vs BL/LL, ENL and TT/BT vs BL/LL, ENL was found to be statistically significant (p < 0.05); whereas the difference between BL/LL and ENL was not found to be statistically significant (p > 0.1) (Table 3).
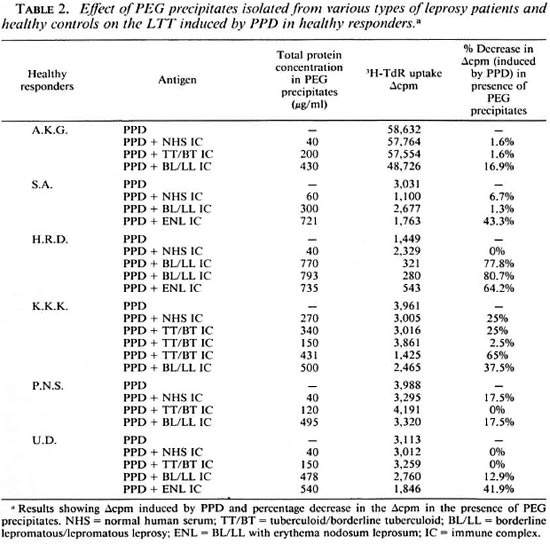
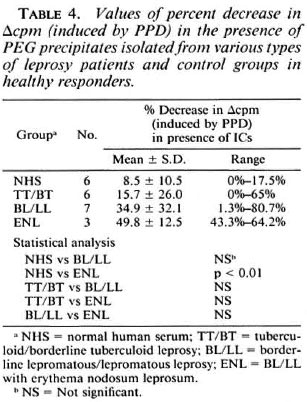
Effect of PEG precipitates on LTT induced by PPD. PEG precipitates from the ENL group were found to exert a statistically significant suppressive effect on the LTT induced by PPD. PEG precipitates from TT/BT, BL/LL and normal control groups, however, failed to show any such effect. The percent decreases in the mean values of Δcpm were: NHS, 8.46 ± 10.47; TT/BT, 15.68 ± 26.0; BL/LL, 34.94 ± 32.10 and ENL, 49.81 ± 12.48. (NHS vs ENL, p < 0.01) (Table 4). However, the PEG precipitates from 2 out of 7 BL/LL patients were found to exert a suppressive effect on the LTT induced by PPD. The percent decreases in their Δcpm were 77.48% and 80.67%, respectively (Table 2).
DISCUSSION
There have been several studies which indicated that in lepromatous leprosy the CM I is depressed specifically to M. leprae, but the mechanism of unresponsiveness of lymphocytes against M. leprae antigens is far from clear. The failure of T lymphocytes to proliferate in response to M. leprae in vitro was, however, interpreted in terms of: a) clonal deletion (27); b) selective defect of presensitized T cells in IL-2 secretion (6, 10) defect in IL-2 receptor expression (11); d) specific T-suppressor cells (9); and e) specific suppressor macrophages (21, 22). In our previous study (28), we showed that patients with BL/LL types of leprosy and patients with ENL have elevated levels of mycobacterial IC(MIC) in their circulation. Whether CICs play any suppressive role in leprosy is, however, not known. There have been reports in which ICs have been shown to act as an immunomodulator (30). In addition, ICs have been shown to enhance (12) as well as to suppress (25) immune responses.
In a mouse model in the recent past, Rao, et al.(17) have shown that pretreatment of H-2b mice with Ag-Ab complexes rendered the mice incapable of mounting an effective immune response to viable allotypic tumor cells. Similarly, our results in an in vitro situation showed a significant suppression of M. leprae-induced lymphocyte proliferation by PEG precipitates obtained from BL/LL and ENL patients. However, PEG precipitates obtained from TT/BT and normal control groups were found to exert no significant suppressive effect on the lymphocyte proliferation. It is possible that the elevated levels of mycobacterial ICs (28) in the BL/LL and ENL patients may have a direct immunosuppressive effect on the LTT induced by CFE. It may also be argued that possibly due to antimycobacterial antibodies in CICs in BL/LL and ENL groups, formed by antibody excess, these antibodies may have bound to M. leprae antigens. This might have lowered the concentration of free M.leprae antigens available in the culture for the optimal stimulation of the lymphocytes.
The other possible explanation for the suppression of lymphocyte proliferation might be due to the presence of elevated levels of immunosuppressive molecules, such as phenolic glycolipid-I (PGL-I) and lipoarabinomannan (LAM), in the sera of these patients (8). However, in our study PEG precipitates (from the BL/LL and ENL groups) alone were found to have no significant effect on the LTT (Fig. 2), indicating thereby that the concentration of PGL-I and/ or LAM (if any) in the complex would be minimal and, hence, may not be optimal for inhibition of the proliferation of the sensitized lymphocytes.
Further, the presence of low or negligible levels of MIC in TT/BT and normal control groups may explain the finding of the insignificant suppressive effect of these PEG precipitates on the lymphocyte proliferation induced by CFE in these patients (Table 3). Similarly, the presence of the insignificant differences in the mean values of MIC levels between BL/LL and ENL groups (28) may explain the finding of insignificant differences in the mean values of percent decreases in Δcpm (induced by CFE) between BL/ LL and ENL groups (Table 3).
It is interesting to note that PEG precipitates from TT/BT and BL/LL groups were found to have no significant suppressive effect on the LTT induced by PPD. However, the significant suppressive effect of PEG precipitates from the ENL group and the depression noted in two patients with BL/ LL leprosy could not be explained from the present study. It is, however, possible that these patients may have elevated levels of MICs that might have induced the suppressive effect.
From these observations, it may be concluded that CICs in leprosy patients have the ability to suppress lymphocyte proliferation induced by M. leprae antigens. However, the exact mechanism of suppression for proliferation of lymphocytes is not clear. Further studies are needed at the molecular level to find out the mechanism for suppression of the LTT responses by CICs in leprosy patients.
Acknowledgment. We thank Dr. R. J. W. Rees, IMMLEP (WHO) Bank, London, and LEPRA U.K. for providing us with the soluble M. leprae antigen and purified protein derivative. We also thank Mr. Hari Om Agarwal and Mr. Neeraj Dube for photographic help. Mr. Anil Kumar Chopra for secretarial assistance, and Mr. P. N. Sharma, Mr. Malikhan Singh and Mr. M. Alam for technical assistance.
REFERENCES
1. BJORVATAN, B., BARNETSON, R. ST. C. KRONVALL, G. K., ZUBLER, R. H. and LAMBERT, P. H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin. Exp. Immunol. 26(1976)388-396.
2. BOYUM, A. Isolation of lymphocytes, granulocytes, and macrophages. In: Lymphocyte IsolationFractionation and Characterization. Natvig, J. B., Perimann, P. and Wigzell, H., eds. London: University Park Press, 1976, pp. 9-15.
3. CLOSS, O., REITAN, L. J., NEGASSI, K., HARHOE. M. and BELEHU, A. in vitro stimulation of lymphocytes in leprosy patients, healthy contacts of 15.leprosy patients, and subjects not exposed to leprosy: comparison of an antigen fraction prepared from M. leprae and tuberculin purified protein derivative. Scand. J. Immunol. 16(1982)103-1 15.
4. FlJRUKAWA. F., YOSHIDA, H., SEKITA, K., OZAKI, M., IMAMURA, S. and HAMASHIMA, Y. Different 16.mode of circulating immune complexes and a.itissDNA antibodies in sera of lepromatous leprosy and systemic lupus erythematous. Lepr. Rev. 55(1984)291-299.
5. GODAL, T., MYKLESTAD, B., SAMUEL. D. R. and 17.M YRVANG, B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin. Exp. Immunol. 9(197 1)821-831.
6. HAREGEWOIN, A., GODAL, T.. MUSTAFA. A. S., BELEHU, A. and YEMANEBERHAN, T. T cell conditioned media reverse T-cell unresponsiveness in 19.lepromatous leprosy. Nature 303(1983)342-444.
7. KAPLAN, G., WEINSTEIN, D. E.. STEINMAN, R. M., LEVIS, W. R., ELVERS, V., PATARROYO, M. E. and COHN, Z. A. Analysis of in vitro T cell responsiveness in lepromatous leprosy. J. Exp. Med. 162(1985)917-929. 20.
8. MEHRA, V., BRENNAN, P. J., RADA, E., CONVIT, J. and BLOOM, B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-196.
9. MEHRA, V., MASON, L. H., FIELDS, J. P. and BLOOM, B. R. Lepromin-induced suppressor cells in patients with leprosy. J. Immunol. 123(1979)1813-1817.
10. MODLIN, R. L., HOFMAN. F. M., HORWITZ. D. A., HUSMANN. L. A., GILLIS, S.. TAYLOR, C. R. and REA, T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin-2 and its receptor. J. Immunol. 132(1984)3085-3090.
11. MOHAGHEGHPOUR, N., GELBER, R. H., LARRICK, J. W.. SASAKI, D. T., BRENNAN, P. J. and ENGLE-MAN, E. G. Defective cell mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to M. leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by IL-2. J. Immunol. 135(1985)1443-1449.
12. MORGAN, E. L. and WEIGLE. W. O. Biological activities residing in the Fc region of immunoglobulin. Adv. Immunol. 40(1987)61-134.
13. NATH, L, JAYARAMAN, T., SATHISH, M., BHUTANI, L. K. and SHARMA. A. K. Inhibition of interleukin-2 production by adherent cell factors from lepromatous leprosy patients. Clin. Exp. Immunol. 58(1984)531-538.
14. NATH, L, SATHISH, M., JAYARAMAN, T., BHUTANI, L. K. and SHARMA, A. K. Evidence for the presence of M. leprae-reactive T lymphocytes in patients with lepromatous leprosy. Clin. Exp. Immunol. 58(1984)522-530.
15. NELSON, D. S., PENROSE, J. M., WATERS, M. F. R., PEARSON, J. M. H. and NELSON, M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions; influence of antileprosy treatment. Clin. Exp. Immunol. 22(1975)385-392.
16. RAMANATHAN, V. D.. PRAKASH, O., RAMU, G., PARKER, D., CURTIS, J., SENGUPTA, U. and TURK, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immunopathol. 32(1984)261-268.
17. RAO, V. S.. BENNETT, J. A.,SHEN, F. W.,GERSHON, R. K. and MITCHELL, M. S. Antigen-antibody complexes generate Lytl inducers of suppressor cells. J. Immunol. 125(1980)63-67.
18. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
19. SAHA, K., CHAKRABARTY. A. K. and PRAKASH, N. A quick method of demonstrating bacillaemia in patients with lepromatous leprosy and ultrastructural studies of the circulating acid fast bacilli. Trans. R. Soc. Trop. Med. Hyg. 77(1983)660-664.
20. SAHA, K., CHAKRABARTY, A.. SHARMA. V. K. and SEHGAL. V. N. Polyethylene glycol precipitates in serum during and after erythema nodosum leprosum - study of their composition and anticomplementary activity. Int. J. Lepr. 52(1984)44-48.
21. SALGAME, P. R., MAIIADEVAN, P. R. and ANTIA, N. H. Mechanism of immune suppression in leprosy: presence of suppressor factors from macrophages of lepromatous patients. Infect. Immun. 40(1983)1119-1126.
22. SATHISH, M., BHUTANI, L. K.. SHARMA, A. K. and NATH. I. Monocyte derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect. Immun. 42(1983)890-899.
23. SERJEANTSON, S. W. and DRY, P. Lymphocytotoxins in leprosy and in asymptomatic hepatitis B virus infection. Clin. Exp. Immunol. 39(1980)289-296.
24. STONER, G. L., MSHANA, R. N., Touw, J. and BELEHU, A. Studies on the defect in cell-mediated immunity in lepromatous leprosy using HLA-D identical siblings. Scand. J. Immunol. 15(1982)33-48.
25. THEOFILOPOULOS, A. N. and DIXON, F. J. The biology and detection of immune complexes. Adv. Immunol. 28(1979)89-220(978 ref.).
26. TOUW LANGENDIJK, E. J. M., WARNDORF VAN DIEPEN, T., HARBOE, M. and BELEHU, A. Relation between anti-Mycobacterium leprae antibody activity and clinical features in borderline tuberculoid leprosy. Int. J. Lepr. 51(1983)305-311.
27. TURK, J. L. and BRYCESON, A. D. M. Immunological phenomena in leprosy and related diseases. Adv. Immunol. 13(1971)209-266.
28. TYAGI, P., RAMANATHAN, V. D., GIRDHAR, B. K.. KATOCH, K., BHATIA, A. S. and SENGUPTA, U. Activation of complement by circulating immune complexes isolated from leprosy patients. Int. J. Lepr. 58(1990)31-38.
29. WEMAMBU, S. N. C, TURK. J. L., WATERS, M. F. R. and REES, R. J. W. Erythema nodosum leprosum; a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
30. WORLD HEALTH ORGANIZATION. The role of immune complexes in disease: report of a WHO scientific group. Geneva: World Health Organization, 1977, pp. 5-58. Tech. Rep. Ser. 606.
1. M.Sc; Research Assistant; Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
2. Ph.D.; Assistant Professor; Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
3. M.D.; Deputy Director; Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
4. M.D.; Assistant Director; Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
5. M.V.Sc, Ph.D.; Deputy Director, Central JALMA Institute for Leprosy, P.O. Box 31, Taj Ganj, Agra 282001, India.
Dr. S. A. Patil's present address is Department of Microbiology, NIMHANS , Bangalore 560029, Karnataka, India.
Received for publication on 16 January' 1992.
Accepted for publication in revised form on 5 August 1992.