- Volume 60 , Number 4
- Page: 570–4
Use of a different buffer system in the phenolic glycolipid-I ELISA
ABSTRACT
By changing the buffer system in the phenolic glycolipid-I (PGL-I) enzyme-linked immunosorbent assay (ELISA) the sensitivity of the test was increased without altering its specificity. Using a Tris-HCl buffer, significant titers of > 1:300 were found in 53.1% of the sera in paucibacillary (PB) and 98.0% of the sera in multibacillary (MB) groups of patients. Titer levels were also significantly increased. In the PB group of patients with Tris-HCl, the highest titer detected was 1:1200; in the MB group of patients, 1:76,800. Through this modification of the buffer system a more sensitive test was obtained thereby increasing the detectable level of PGL-I antibodies in both the PB and MB groups of patients.RÉSUMÉ
En changeant le système tampon du test enzymatique (ELISA) basé sur le glycolipide phénolique-I (PGL-I), la sensibilité du test a été augmentée sans que sa spécificité ne soit modifiée. En utilisant un tampon Tris-HCl, des titres significatifs > 1:300 ont été trouvés dans 53.1% des scrums de patients paucibacillaires (PB) et 98.0% des scrums de multibacillaires(MB). Les titres étaient également augmentés de manière significative. Dans le groupe des patients PB, avec le Tris-HCl, le titre le plus élevé détecté fut 1:1200; il fut de 1:76,800 dans le groupe des patients MB. Par cette modification du système tampon, on obtint un test plus sensible, augmentant ainsi la capacité de détection des anticorps anli-PGL-I dans les deux groupes de patients PB et MB.RESUMEN
Utilizando un regulador de Tris-HCl, en lugar del clásico regulador de salina fosfatos (PBS), se logró incrementar la sensibilidad del inmunoensayo enzimático (ELISA) para el glicolópido fenólico-I (PGL-I). Con en regulador de Tris-HCl se encontraron títulos significativos de > 1:300 en el 53.1% de los sueros de pacientes paucibacilares (PB) y en el 98% de los sueros de pacientes multibacilares (MB). Los niveles de los títulos también se incrementaron significativamente. Mientras que en el grupo de pacientes PB el título más alto fue de 1:2000, en el grupo de pacientes MB éste fue de 1:76,800. La modificación en el sistema de regulador incrementó la sensibilidad del ensayo sin alterar su especificidad.Since the mid-1980s, the highly specific Mycobacterium leprae phenolic glycolipid-I (PGL-I) antigen has been used extensively in the serological diagnosis of leprosy (9, 13, 19). To detect the predominantly IgMclass antibodies, an enzyme-linked immunosorbent assay (ELISA) was developed using a semisynthetic substance PGL-I disaccharide-bovine serum albumin (D-BSA) as antigen (6, 12). This PGL-I ELISA is used to detect IgM antibody levels in patients with different forms of leprosy, in contacts, and in healthy individuals originating from leprosy-endemic areas (4, 8). Most patients with the lepromatous form of leprosy show high levels of antibodies to PGL-I (l4). Findings among the tuberculoid and indeterminate forms of leprosy are variable (15). A proportion of contacts and healthy individuals from leprosy-endemic areas may also develop antibodies to this antigen (1). The limitations of and objections to the PGL-I ELISA have been voiced previously (3, 5. 11). Likewise, one also may not be able to carry out this test in most laboratories of national leprosy programs. Reference laboratories specializing in leprosy work, however, should be able to perform this test to provide additional information in the diagnosis of leprosy and in different epidemiological settings.
In this study, the previously used (2) phosphate-buffered saline (PBS) buffer in the PGL-I ELISA was replaced with a Tris-HCl buffer. The different antibody levels obtained by the two buffer systems were compared, and the results are presented.
MATERIALS AND METHODS
Two-hundred-forty-five sera were taken from 33 patients at the Departmento de Lepra, Asunción, Paraguay (The Table). Each patient included in this study was classified clinically and histologically. Blood samples were drawn at various times. Skin biopsies were taken at the beginning of multidrug therapy. The sera and biopsies were shipped to the Armauer Hansen Institute (AHI), Wiirzburg, Germany, where the sera were stored at - 70ºC until used. Forty-nine sera from apparently healthy individuals in Paraguay and 89 sera from young students and blood donors in Germany were taken and included in this study. In addition, 21 sera from German patients suffering from syphilis (N = 6), Lyme disease (N = 8), and rheumatoid arthritis (N = 5) were also tested in the PGL-I ELISA.
Histological preparations of skin biopsies
Skin biopsies were immersed in buffered formaldehyde at the Departmento de Lepra, Asunción, Paraguay. The biopsies were cut at the Armauer Hansen Institute and various sections were stained with hematoxylin and cosin (H&E). Fite-Faraco staining for the demonstration of acid-fast bacilli (AFB) was also carried out. Leprosy was classified according to Ridley and Jopling (lfi). In this study, for serological purposes, the histological subclassifications (LLs and LLp) were not used in the lepromatous group of patients; they were all called LL.
Serological assays
PBS buffer. D-BSA and BSA were kindly donated by Dr. H. D. Engers of the IMMLEP Program of the World Health Organization. U-bottom, polystyrene, microliter plates (Greiner No. 650061) were coated with both substances diluted 1:4000 in carbonate-bicarbonate buffer (pH 9.6). The plates were incubated at 4ºC overnight. The next day the plates were washed three times with PBS (Na2HP04 90.75 mmol, NaH2P0 4 22.01 mmol, NaCl 10 mmol) (pH 7.4) containing 0.5% v/v Tween 20 (PBST). The wells were subsequently blocked with 1% w/v BSA (Behring) in PBST. Dilutions of the patients' sera and of the healthy controls' sera were initially carried out at 1:200 and added to the wells. Positive sera were diluted further up to 1:12,800. The sera were diluted in PBS-0.1% v/v Tween 20-10% normal goat serum (NGS) (Gibco No. 200-621 AG). The plates were incubated at 37ºC for 1 hr. After washing the wells three times with PBST, antihuman IgM-peroxide (Dako Code P322-02) diluted 1:5000 in PBS-0.1 % v/v Tween 20-10% NGS was added to the wells. The plates were incubated at 37ºC for 1 hr. After washing the wells three times with PBST, four tablets of 1,2 phenylenediamine (OPD; Dako S2000) were dissolved in 12 ml 0.1 M citric acid (0.035 mole) phosphate buffer (0.067 mole) (pH 5), to which 5 µl 30% H2O2 was added, and this mixture was added to the wells. The plates were incubated at room temperature for 5 min. The reaction was stopped with 2 NH2S04 , and the absorbance was read at 490 nm. Each test was performed in duplicate. The mean absorbance of the wells with BSA was subtracted from one of the wells with D-BSA. Six wells in each plate were allocated for the positive control sera and six wells in each plate for the negative sera. The criteria of seropositivity were determined as previously reported (OD > 0.200) (8).
Tris-HCl buffer. The ELISA was done essentially in the same way as above. However, some modifications in the buffer system were carried out. The PBS solutions were replaced with Tris-HCl. The initial dilutions of the sera from healthy controls and patients were carried out at 1:300, and the positive sera were diluted further up to 1:76,800.
Preparations of solutions. Tris-HCl stock: 0.5 M Tris (Merck No. 8382) = 60.6 g Tris was diluted in 800 ml double-distilled H.O and with 25% HC1 adjusted to a volume of 1000 ml (pH 7.4); 3.5 M NaCl solution was prepared in double-distilled H2O. A wash solution of 100 ml of 3.5 M NaCl, 100 ml Tris-HCl stock (pH 7.4) and 1 ml Tween 20 was added to 1000 ml double-distilled H2O. Serum dilutions were carried out in Tris-HCl-1% NGS. Tris-HCl was prepared as for the wash solution. After addition of the substrate solution to the wells (see above), the plates were put in the dark at room temperature for about 15 min. The reaction was stopped with 2 N H2 S04 , and the absorbance was read at 490 min.
Cut-off value. A serum dilution was considered positive if it yielded a net absorbance greater than the total derived by adding 3 standard deviations to the mean (> X + 3 S.D.) of the absorbance for a group of six negative controls. The cut-ofT value was determined by each run in each plate.
RESULTS
In 26 out of 49 sera taken from paucibacillary (PB) patients an antibody titer > 1:300 was found to PGL-I using Tris-HCl, the highest titer being 1:1200 (Fig. 1). Comparing this result with the titer found in PBS only, three sera showed a titer equal to 1:200 (Fig. 1). This was also the highest titer obtained in the PB group of patients using PBS. The sensitivity of the ELISA using Tris-HCl was 53.1% compared to 6.1% found in PBS in this group of patients. Fifty-seven sera taken from borderline lepromatous (BL) patients were also tested with Tris-HCl. The highest titer obtained was 1:38,400, and 98.3% of the sera tested had a titer > 1:300 (Fig. 1). In the LL group of patients 139 sera were tested with Tris-HCl. The highest titerobtained was 1:76,800, and 97.8% of thesera tested showed a titer > 1:300 (Fig. 1).
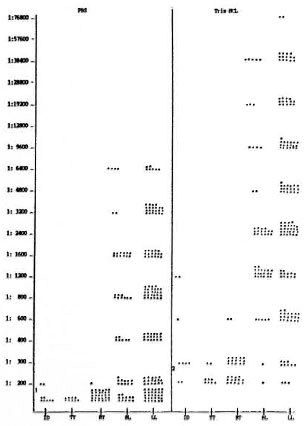
Fig. 1. Comparison of antibody titers between PBSs and Tris-HCI. 1 = in PBS titer, < 1:200 was taken as negative; 2 = in Tris-HCI titer, < 1:300 was taken as negative; ID = indeterminate; TT = tuberculoid; BT = borderline tuberculoid; BL = borderline lepromatous; LL = lepromatous leprosy.
Using the PBS buffer in the sera takenfrom BL patients, the highest titer detectedwas 1:6400, and 79.0% of the sera had atner > 1:200. In the LL group of patientsusing PBS, the highest titer obtained was1:6400, and 78.4% of the sera tested had atiter > 1:200 (Fig. 1). However, taking 1:300as a significant titer as was done in Tris-HC1, the sensitivity of the test with PBSwould have been reduced further.
The overall sensitivity detected in thePGL-I ELISA using Tris-HCl in the PB patients'sera was 53.1% (> 1:300) comparedto 6.1% using PBS (> 1:200). In the multibacillary (MB) patients' sera, the overallsensitivity in Tris-HCl was 98.0% (> 1:300);in PBS, 78.6% (> 1:200).
It was surprising to find antibody levels as high as 1:1200 in patients with the indeterminate type of leprosy using Tris-HCl. Unfortunately, the number of PB patients tested was rather small (N = 9).
Eighty-nine sera from healthy, young German adults were tested in PBS and Tris HC1, and the 98th percentile was chosen as the cut-offlevel. However, it was found that the optical density (OD) value in PBS were generally low, ranging between 0.057-0.095 pare d to Tris-HCl (0.157-0.198). Tris HCl gave a much more satisfactory color reaction than did PBS (see positive controls, Fig. 2), and the results were much more reproducible. Therefore, it was decided not to take the OD value of > 0.200 as the cut off used in PBS, but to calculate it for each run on the basis of six negative controls (> X + 3 S.D.). The Tris-HCl cut-off value rarely exceeded 0.200, and when it did, the run was repeated.
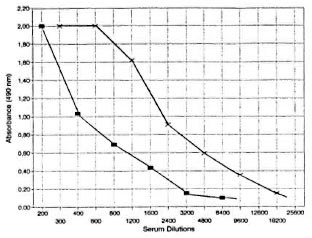
Fig. 2. Comparison of OD values of positive sera tested in PBS; and Tris-HCI. -■- = PBS; -×- = Tris-HCI.
Forty-nine sera from healthy, young Par aguayans who were not household contacts of known leprosy patients were also tested. No essential differences could be detected between the different control groups.
Sera tested in a PGL-I ELISA from pa tients suffering from syphilis, Lyme disease, and rheumatoid arthritis did not show any antibodies to PGL-I. This testing was car ried out to exclude a possible interference with other IgM-class antibodies (rheuma toid factor being an IgM type antibody), and thereby altering the specificity of the PGL-I ELISA.
DISCUSSION
In the PGL-I ELISA carried out in this study the buffer system was changed. By replacing PBS with the Tris-HCl a higher degree of sensitivity was detected without changing its specificity; 53.1% of the sera in the PB group of patients showed significant antibody levels (> 1:300) versus 6.1% using PBS (> 1:200). In the MB group of patients using Tris-HCl a significant titer was found in 98.0% of the sera tested compared to 78.6% found with PBS. The highest titer obtained in the indeterminate, tuberculoid (TT), and borderline tuberculoid (BT) groups of patients using PBS was 1:200; with Tris-HCl, 1:1200. In the BL type of leprosy the highest titer using Tris-HCl was 1:38,400; using PBS only, it was 1:6400. In the LL form an even higher titer of 1:76,800 was noted using Tris-HCl. Using PBS in both the BL and LL groups of patients, the highest titer was 1:6400 (Fig. 1).
At the present time very few serological tests are available for the detection of M. leprae-specific antibodies (l8). The PGL-I ELISA is still of considerable diagnostic importance ( 7, 10, 17 ) . A more sensitive PGL-I ELISA-as described here -may provide a more useful tool for the serological diagnosis of leprosy.
In conclusion, the sensitivity of the PGL-I ELISA has been increased by changing the buffer system. Through this modification the PGL-I ELISA became a more sensitive serological test for the detection of M. leprae-specific antibodies in the sera of both paucibacillary and multibacillary patients.
Acknowledgment. We would like to thank Miss S. Hübncr. Mrs. D. Jaschinski, and Mrs. C. Gachter for their excellent technical assistance.
REFERENCES
1. BAUMGART, K., BRITTON, W., BASTEN, A. and BAGSUAWE, A. Use of phenolic glycolipid I for scrodiagnosis of leprosy in a high prevalence village in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 81(1987)1030-1032.
2. BRETT. S. J., PAYNE, S. N., GIGG, J., BURGESS, P. and GIGG, R. Use of synthetic glycoconjugates containing the Mycobacterium leprae specific and immunodominant epitope of phenolic glycolipid I in the serology of leprosy. Clin. Exp. Immunol. 64(1986)476-483.
3. CARTEL, J.-L., CHANTEAU, S., BOUTIN, J.-P., PLICHART, R., RICHEZ, P., Roux, J.-F. and GROSSET, J.-H. Assessment of anti-phenolic glycolipid-I IgM levels using an ELISA for detection of M. leprae infection in populations of the South Pacific Islands. Int. J. Lepr. 58(1990)512-517.
4. CHANTEAU, S., CARTEL, J.-L., GUIDI, C , PLI-CHART, R. and BACH, M.-A. Seroepidemiological study on 724 household contacts of leprosy patients in French Polynesia using disaccharide-oclyl-BSA as antigen. Int. J. Lepr. 55(1987)626-632.
5. CHANTEAU, S., CARTEL, J.-L., PERANT, E., N'DELI, L., Roux, J. and GROSSET, J.-H. Relationship between PGL-1 antigen in serum, tissue and viability of Mycobacterium leprae as determined by mouse footpad assay in multibacillary patients during short-term clinical trial. Lepr. Rev. 61(1990)330-340.
6. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H., ASPINALL, G. O. and BRENNAN, P. J. Use of an artificial antigen containing 3,6-di-0-methyl-β-D-glucopyranosyl epitope for the scrodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
7. CHO, S.-N., KIM, S.-H., CELLONA, R. V., CHAN, G. P., FASARDO, T. T., WALSH, G. P. and KIM, J. D. Prevalence of IgM antibodies to phenolic glycolipid I among household contacts and controls in Korea and The Philippines. Lepr. Rev. 63(1992)12-20.
8. CHO, S.-N., SHIN, J.-S., CHOI, I. H., KIM, D. I. and KIM, J.-D. Detection of phenolic glycolipid-I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsei Med. J. 29(1988)219-224.
9. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
10. DOUGLAS, J. T., CELLONA, R. V., ABALOS, R. M., MADARANG, M. G. and FAJARDO, T. Serological activity and early detection of leprosy among contacts of lepromatous patients in Ccbua, The Philippines. Int. J. Lepr. 55(1987)718-721.
11. FINE, P. E. M., PONNINGHAUS, J. M., BURGESS, P., CLARKSON, J. A. and DRAPER, C. Seroepide miological studies of leprosy in northern Malawi based on an enzyme-linked immunosorbent assayusing synthetic glycoconjugate antigen. Int. J. Lepr. 56(1988)243-254.
12. FUJIWARA, T., HUNTER, S. W., CHO, S.-N., ASPIN ALL, G. O. and BRENNAN, P. J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for scrodiagnosis of leprosy. Infect. Immun. 43(1984)245-252.
13. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1982)728-735.
14. KOSTER, F. T., SCOLLARD, D. M., UMLAND, E. T., FISHBEIN, D. B., HANLY, W. C, BRENNAN, P. J. and NELSON, K. E. Cellular and humoral immune response to a phenolic glycolipid antigen (phen Gl-I) in patients with leprosy. J. Clin. Microbiol. 25(1987)551-556.
15. LYONS, N. F., SHANNON, E. J., ELLIS, B. P. B. and NAAFS, B. Association of IgG and IgM antibodies to phenolic glycolipid-I antigen of Mycobacterium leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
16. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
17. ROCHE, P. W., THEUVENET, W. J. and BRITTON, W. J. Risk factors for type-1 reactions in borderline leprosy patients. Lancet 1(1991)654-656.
18. SENGUPTA. U. Mycobacterium leprae ANTIGENSAND their utility in immunodiagnosis of leprosy. Trop. Med. Parasitol. 41(1990)361-362.
19. YOUNG, D. B. and BUCHANAN, T. M. A serological lest for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. M.D., F.R.C.P.(C).; Appl. Professor of Microbiology, Armauer Hansen Institute/DAHW, Hermann-Schell Str. 7, 8700 Wurzburg, Germany.
2. Dr.; Armauer Hansen Institute/DAHW, Hermann-Schell Str. 7, 8700 Wurzburg, Germany.
3. Dr.; Director, Departamento de Lepra. Asunción. Paraguay.
4. Departamento de Lepra. Asunción. Paraguay.
Received for publication on 12 May 1992.
Accepted for publication in revised form on 8 October 1992.