- Volume 60 , Number 4
- Page: 575–9
Microliter particle agglutination test for diagnosis of leprosy
ABSTRACT
The results of studying the microtiter particle agglutination (MPA) test for detecting asAi-Mycobacterium leprae antibodies in blood sera are presented. The sero diagnostic test is based on the agglutination of colored polyacrolein latex microparticles (PAMP) conjugated with 3,6-di-O-methylD-glucose (DMG). Sera f rom 45 leprosy patients (LL, BL), 34 leprosy contacts, and 148 control subjects were investigated by the MPA test. A correlation between the anti-M. leprae antibodies and the bacterial load was found. In many long-treated leprosy patients increased titers of anti-DMG antibodies were observed, which might be due to specific polyneuritis in them. Four contacts of leprosy patients were also positive in the MPA test. "Nonleprosy" sera did not react in the test. The method proposed proved to be of high specificity and sensitivity for the serological diagnosis of leprosy. The rapidity, simplicity, and visual assessment of the results allow the method to be used in the field for epidemiological studies of leprosy contacts and the general population in leprosy-endemic areas.RÉSUMÉ
Les résultats de l'étude du test d'agglutination de microparticules (MPA) pour la détection d'anticorps anti-Mycobacterium leprae dans le sérum sont présentés. Le test sérodiagnostique est basé sur l'agglutination de microparticules de polyacroléine de latex colorées (PAMP) conjuguées au 3,6-di-O-methyl-D-glucose (DMG). Le scrum de 45 malades de la lèpre (LL, BL), 34 contacts, et 148 témoins a été analysé par le test MPA. On a trouvé une correlation entre les anticorps anti-M. leprae et la charge bactérienne. On a observé chez de nombreux patients ayant suivi un traitement de longue durée une augmentation des titres d'anticorps anti -DMG, ce qui pourrait être du à la présence d'une polynévrite spécifique. Quatre contacts de malades de la lèpre étaient également positifs pour le test au MPA. Le scrum des personnes "non-lépreuses" n'a pas réagi au test. La méthode proposée s'est montrée d'une spécificité et d'une sensibilité élevées pour le diagnostic sérologiquc de la lèpre. La rapidité, la simplicité et le controle visuel des résultats permettent à la méthode d'être utilisée sur le terrain pour des études épidémiologiques portant sur des contacts de malades de la lèpre et la population générale dans les régions endémiques.RESUMEN
Se presentan los resultados sobre la prueba de aglutinación de partículas (PAP) para detectar anticuerpos mú-Mycobacterium leprae en el suero sanguíneo. La prueba se basa en la aglutinación de partículas coloridas de látex-poliacrolcína, conjugadas con 3.6-di-O-metil-D-glucosa (DMG). Con esta prueba se analizaron los sueros de 45 pacientes con lepra (LL, BL). de 34 contactos de los pacientes, y de 148 individuos control. Se encontró una correlación entre los anticuerpos anti- M. leprae y la carga bacteriana. En muchos pacientes con lepra con muchos años de tratamiento se observaron títulos aumentados de anticuerpos anti-DMG. Esto podría estar relacionado con la polineuritis específica que presentan estos pacientes. Cuatro contactos de los pacientes con lepra también fueron positivos en la preuba de aglutinación de partículas. Los sueros de las personas no relacionadas con la enfermedad no dieron reacciones positivas. El método propuesto para el diagnóstico serológico de la lepra, resultó ser de alta especificidad y sensibilidad. La rapidez, simplicidad. y la evaluación visual de los resultados, permiten que el método pueda ser usado en estudios epidemiológicos de campo en las áreas donde la lepra es endémica.Nowadays the scrodiagnosis of leprosy can be improved significantly by using highly purified specific antigens such as phenolic glycolipid I (PGL-I) from cell walls of Mycobacterium leprae, the specificity of which is determined by the terminal sugar moiety 3,6-di-O-methyl-D-glucose (4, 7, 9).
The carbohydrate analogs of PGL-I were synthesized and proved to be useful in immunoenzyme assays for detection of specific antibodies in sera from leprosy patients and their household contacts (2, 5). Yet the diagnosis of leprosy in the field, especially in remote and not easily accessible areas, is preferred to be done with simple, noninstrumental methods (for example, agglutination tests) not requiring any special laboratory equipment.
Recently, reports have appeared on the possibility of the rapid serodiagnosis of leprosy on the basis of agglutination reactions using latex (16) and gelatin (9) particles.
This paper is devoted to the development of a microtiter particle agglutination (MPA) test for the serodiagnosis of leprosy on the basis of colored polyacrolein microparticles (10) conjugated with β-(3-aminopropyl)3,6di-0-methyl-D-glycopyranoside (DMG) (1), a synthetic analog of a specific carbohydrate epitope of PGL-I. Earlier it was shown that DMG conjugated with bovine serum albumin (BSA) might be useful in an ELISA for the serodiagnosis of leprosy (19).
MATERIALS AND METHODS
Sera. Blood sera from 45 leprosy patients (lepromatous, borderline lepromatous), 34 contacts of leprosy patients, 6 persons with suspected leprosy, 55 tuberculosis patients, 13 subjects with acute infectious diseases (epidemic meningitis, viral hepatitis, salmonellosis, dysentery), and 74 healthy donors were studied.
Latex conjugate preparation. DMG was synthesized as described earlier (1)- Colored polyacrolein microparticles (PAMP; 1.4 µm in diameter) were obtained by aqueous polymerization of acrolein under alkaline conditions in the presence of pyronin G (10); 0.1 ml of DMG was added to 1 ml of 3% (w/ v) PAMP suspension in 0.15 M phosphatebuffered saline (PBS), pH 7.4, and the mixture was shaken gently for 2 hr at 20ºC. Unrcacted DMG was removed by centrifugation (300 × g × 5 min), and the pellet was washed three times with PBS suspended in 1% glycine solution containing 0.15 M NaCl (GBS), pH 8.6, allowed to stand for 2 hr, and finally resuspended in 1 ml of PBS with 0.01% sodium azide.
Microtiter particle agglutination (MPA) test. Before testing, the serum samples were diluted 1:25 with PBS, then heat-inactivated at 56ºC for 30 min. Microtiter plates with U-bottom wells (Dynatech, Chantilly, Virginia, U.S.A.) were used for the MPA tests. Twofold serial dilutions of the test sera (50 µl) in GBS containing 0.1% BSA (GBS-BSA) were prepared in the microplate wells. A 3% stock solution of PAMP-DMG was diluted to 0.3% with GBS-BSA, and 25 µl of PAMP-DMG was added to each well containing the titered sera. Two negative wells with a) unconjugated PAMP + test scrum (1:50), and b) PAMP-DMG + GBS-BSA were run in each assay. For the proper interpretation of the MPA results, both control wells should show a negative reaction (distinct pellet on the bottom of the well). The results of the reaction were scored on a 4+ scale after a 2-hr incubation at 20ºC, with regard for the end point of a positive reaction in the last well showing 2+ agglutination.
ELISA. The U-wells of the polystyrene plates were coated with DMG-BSA (0.001 mg/ml) in carbonate-bicarbonate buffer (pH 9.6), incubated overnight at 20ºC, and then washed with PBS containing 0.05% Tween 20 (PBS-Tween).
Nonspecific binding was blocked with 1% BSA. The plates were washed with PBS-Tween, covered with serum samples (0.1 ml) diluted to 1:200 in PBS containing 1% fetal calf serum (FCS), and incubated for 2 hr at 20ºC. Again, the plates were washed with PBS-Tween and incubated with rabbit antihuman IgM antibodies-peroxidase conjugate (Sevac, Czechoslovakia) diluted to 1:2000 for 1 hr at 37ºC. The plates were then washed with PBS, a mixture of orthophenylene diamine and hydrogen peroxide in citrate-phosphate buffer was added as a substrate, and the plates were incubated at 37ºC for 1 hr. The reaction was stopped by adding 2.5 N sulfuric acid and read in a Minireader (Dynatech) at 490 nm. The results were expressed in units of optical density (OD) as corrected for background values. The criterion of positivity was established as OD > 0.20.
RESULTS AND DISCUSSION
The results of the MPA test are presented in Figure 1. In the patients with active leprosy (newly discovered and relapsed) antibodies against DMG were demonstrated at the final scrum dilutions of 1:800-l :25,600. A direct correlation between anti-DMG antibody titer and the bacterial load was not always obvious. This may be accounted for by the variations in individual responses to M. leprae antigens in leprosy patients (8, 14, 15). Twenty-five out of 30 patients with long-treated leprosy consistently showed increased levels of DMG-antibodies (1:400). This may be due to the persistence of mycobacteria in the tissues and peripheral nerves of these patients (12-14, 17). The clinical examination of such patients revealed signs of specific polyneuritis. In the remaining five patients from this group the titers of antibodies against DMG did not exceed 1:100.
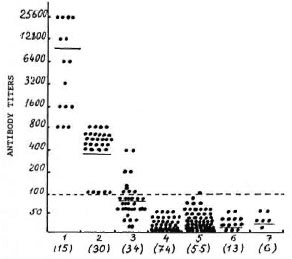
Fig. 1. Serum antibody titers against 3,6-dimethyl glucose (DMG) in MPA test: l = active leprosy patients; 2 = long-treated leprosy patients; 3 = healthy contacts of leprosy patients; 4 = otherwise healthy persons ("donors"); 5 = tuberculosis patients; 6 - patients with other infectious diseases; 7 = persons with suspected leprosy. Figures in parentheses = number of subjects under investigation. Each point represents one individual serum; mean values are indicated by horizontal lines; dotted line = cut-off point for seropositivity.
Most of the "nonleprosy" sera did not react with PAMP-DMG or were positive at the dilution of 1:50. Only one serum from a patient with tuberculosis gave a positive reaction at a 1:100 dilution. The dilution of 1:200 was accepted by us as a cut-off point for diagnosis because at this dilution none of the control sera was positive and the sera from leprosy patients gave 2+ reactions. In connection with this finding, the results obtained in leprosy contacts are rather interesting. In the group of leprosy contacts four persons had anti-DMG antibodies at 1:200 and 1:400 dilutions which permitted us to include them in a group at risk of developing leprosy.
Analysis of the sera using ELISA with DMG-BSA as an antigen showed a high correlation with the MPA results for all of the groups studied (r = 0.73; p < 0.01) (Fig. 2), although some overlapping was noted. At the same dilution, sera which were positive in MPA gave different OD values although they were all > 0.20. MPA-negative sera (titer 1:100 or less) gave uniformly low values in ELISA (OD < 0.20), with the exception of two sera from the patients with longtreated leprosy giving OD values of > 0.20, but negative results in MPA at a dilution of 1:100, and one serum from a leprosy contact, which was positive in MPA (1:200) and negative in the ELISA (OD < 0.20). We think it advisable to consider more carefully MPA-positive results at dilutions of 1:100 and 1:200 in leprosy contacts and in the general population of hyper-endemic areas with a view of avoiding overdiagnosis or underdiagnosis. It is well known that in leprosy-endemic areas regular follow up of seropositives does not always discover increased levels of anti-M. leprae antibodies, and the disease does not always develop in seropositive persons (3, 6, 11). In our opinion, all seropositive persons are at risk of developing leprosy and should be kept under surveillance with regular MPA tests at least twice a year.

Fig. 2. Comparative results of the detection ofanti-DMG antibodies in sera with the MPA test and ELISA (see Fig. 1 legend for details).
Our results are in good concordance with Wu, et al.'s data (17), but we consider that our MPA method has certain advantages over the one proposed by them in that the way of binding hapten (DMG) with a carrier (PAMP) provides a higher stability and higher capacity of the conjugate. In addition, titration of sera by our method increases the sensitivity and demonstrability of the reaction, while the use of the whole sera in latex agglutination slides might give false-negative results due to excess antibodies ("prozone" phenomenon).
Thus, the microtiter particle agglutination test proposed for the detection of M. leprae-specific antibodies and based on a latex conjugate with DMG as an antigen proved to be rather sensitive, highly specific, and showed good correlation with the ELISA. The advantages of the MPA test are: rapidity (1.5-2 hr), thus escaping multistep performance, and noninstrumental assessment of the results, making it especially useful in seroepidemiological studies on household contacts of leprosy patients and healthy persons in leprosy-endemic areas.
Acknowledgment. We greatly appreciate the assistance of Mrs. V. Rezacva in preparation of the manuscript.
REFERENCES
1. BOVIN, N. V., KORCHAGINA, E. Y., ZEMLYA-NUIKHINA, T. V., BAIRAMOVA, N. E., GALANINA, O. E., ZEMLYAKOV, A. E., IVANOV, A. E., ZUBOV, S. P. and MOCHALOVA, L. V. Synthesis of polymeric neoglycoconjugates. J. Glycoconjug. (1992) (in press).
2. BRETT, S. J., PAYNE, S. N., GIGG, J., BURGESS, P. and GIGG, R. Use of synthetic glycoconjugates containing the Mycobacterium /cpraospecific and immunodominant epitope of phenolic glycolipid-I in the serology of leprosy. Clin. Exp. Immunol. 64(1988)476-483.
3. CHANTEAU, S., CARTEL, J.-L., ROUX, J., PLICHART, R. and BACH, M.-A. Comparison of synthetic antigens for detecting antibodies to phenolic glycolipid-I in patients with leprosy and their household contacts. J. Infect. Dis. 157(1988)770-775.
4. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of an artificial antigen containing the 3,6-di-O-methyl-β-D-glucopyranosyl epitope for serodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
5. CHO, S.-N., YANAGIHARA, D. L., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1084.
6. DOUGLAS, J. T., CELLONA, R. V., ADALOS, R. M., MADARANG, M. G. and FAJARDO, T. The serological reactivity and early detection of leprosy among the contacts of lepromatous patients in Ccbu, Philippines. Int. J. Lepr. 55(1987)718-721.
7. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
8. HUSSAIN, R., JAMIL, S., KIFAYET, A., FIRDAUSI, F., DOCKRELL, H. M., LUCAS, S. and HASAN, K. Quantitation of IgM antibodies to the M. leprae synthetic disaccharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58(1990)491-502.
9. IZUMI, S., FUJIWARA, T., IKEDA, M., NISIIIMURA, Y., SUGIYAMA, K. and KAWATSU, K. Novel gelatin particle agglutination test for serodiagnosis of leprosy in the field. J. Clin. Microbiol. 28(1990)525-529.
10. LUKIN, Y. V., BAKHAREV, V. N., ZAICHENKO, A. S., VORONOV. S., ZUBOV, V. P., GRITSKOVA, I. A. and PRAVEDNIKOV, A. N. [Polyacrolcin latexes: synthesis, filling and formation.] Trans. Acad. Sci. U.S.S.R. 285(1985)159-161.
11. MENZEL, S., HARBOE, M., BERGSVIK, H. and BRENNAN, P. J. Antibodies to a synthetic analog of phenolic glycolipid-I of Mycobacterium leprae in healthy contacts of patients with leprosy. Int. J. Lepr. 55(1987)617-625.
12. MILLER, R., HARNISCH, J. and BUCHANAN, T. Antibodies to mycobacterial arabinomannan in leprosy: correlation with reactional states and variation during treatment. Int. J. Lepr. 52(1984)133-139.
13. MSHANA, R. N., HUMBER, D. R., HARBOE, M. and BELEHU, A. Demonstration of mycobacterial antigens in nerve biopsies from leprosy patients using peroxidase-antiperoxidasc immunoenzyme technique. Clin. Immun. Immunopathol. 29(1983)359-368.
14. ROCHE, P. W., BRITTON, W. J., FAILBUS, S. S., LUDWIG, H., THEUVENET, W. J. and ADIGA, R. B. Heterogeneity of serological responses in paucibacillary leprosy-differential responses to protein and correlation with clinical parameters. Int. J. Lepr. 58(1990)319-328.
15. ROCHE, P. W., BRITTON, W., FAILBUS, S. S., WILLIAMS, D., PRADHAN, H. M. and THEUVENET, W. J. Operational value of serological measurement in multibacillary leprosy patients: clinical and bacteriological correlates of antibody responses. Int. J. Lepr. 58(1990)480-491.
16. WABITSCH, K. R. and MEYERS, W. M. Histopathologic observations on the persistence of Mycobacterium leprae in the skin of multibacillary leprosy patients under chemotherapy. Lepr. Rev. 59(1988)341-346.
17. Wu, Q., YE, G.-Y., YIN, Y.-P., LI, X.-Y., LIU, Q. and WEI, W .-H. Rapid serodiagnosis for leprosy-preliminary study on latex agglutination test. Int. J. Lepr. 58(1990)328-333.
18. YOUNG, D. 13. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
19. YUSCHENKO, A. A., BOVIN, N. V., SUKHENKO, L. T., URLYAPOVA, N. G., BAIRAMOVA, N. E. and DYACHINA, M. N. Use of artificial antigens with M. leprae-PGL-] properties for the studies of leprosy. (Abstract) Int. J. Lepr. 57 Suppl. (1989)409.
1. Dr.Med.; Senior Scientific Worker, Leprosy Research Institute, GSP-7, Astrakhan 41400, Russia.
2. Dr.Chem.; Senior Scientific Worker; Shemyakin's Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia.
3. Dr.Chem.; Professor, Head, Polymers for Biology Laboratory; Shemyakin's Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia.
4. Dr.Chem.; M. M. Shemyakin's Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, Russia.
Received for publication on 18 May 1992.
Accepted for publication on 28 July 1992.