- Volume 60 , Number 4
- Page: 580–6
Cold fingers in leprosy
ABSTRACT
Under conditions of maximal thermoregulatory peripheral dilatation, most healthy subjects (both Indian and European) showed raised blood flow in the fingertips (measured by laser Doppler flowmetry) where the skin temperature is only slightly lower than the core body temperature. Most borderline lepromatous (BL) leprosy patients had much colder fingers and the blood flow was slow: borderline tuberculoid (BT) patients had skin temperatures similar to those seen in healthy subjects, but their fingertip blood flow was reduced relative to that in control subjects. The occurrence of cold fingers and slow blood flow was clearly associated with evidence of sensory impairment to light touch, pressure and temperature. Slower fingertip blood flow was strongly associated with impairment of vasomotor control in this anatomical region, suggesting that both may be a consequence of leprosy peripheral neuropathy, at least in patients with early leprosy, but it is likely that leprosy arteriopathy may contribute to the lowered peripheral perfusion in advanced cases. It is suggcsted that the simple clinical sign of cold iingers may be of value in the prcliminary assessment of patients presenting at any leprosy control clinic in the tropics.RÉSUMÉ
Dans des conditions de dilatation périphérique maximale par thermorégulation, la majorité des personnes en bonne santé (aussi bien des Indiens que des Européens) a montré une augmentation du flux sanguin (mesuré par effet Doppler) dans le bout des doigts, où la température cutanée est seulement légèrement plus basse que la température interne. La majorité des patients atteints de lèpre borderlinc lépromateuse (BL) avait des doigts beaucoup plus froids, et la circulation sanguine était plus lente; les patients ayant une lèpre borderlinc tuberculoide (BT) avaient une température cutanée semblable à celle des individus en bonne santé, mais leur flux sanguin au bout des doigts était relativement réduit par rapport aux témoins. La présence de doigts plus froids et d'un flux sanguin ralenti était clariement associée à une détérioration de la sensibilité tactile légère, ainsi que de la sensibilité à la pression et à la température. Le ralentissement du flux sanguin au bout des doigts était clairement associé à une détérioration due controle vasomoteur dans cette région anatomique, suggérant que tous deux peuvent être la conséquence d'une neuropathic périphérique lépreuse. au moins chez les patients avec une lèpre débutante; il est cependant vraisemblable qu'une artériopathie lépreuse puisse contribuera la diminution de la perfusion périphérique dans les cas avancés. La suggestion est faite que le signe clinique simple des doigts froids puisse avoir de la valeur dans l'examen préliminaire des patients se présentant à une consultation de lèpre sous les tropiques.RESUMEN
Bajo condiciones de máxima dilatación periférica lermorcguladora, la mayoría de los sujetos sanos (indues y europeos) mostraron elevado flujo sanguíneo en la punta de los dedos (medido por flujometría laser de Doppler), donde la temperatura de la piel es sólo ligeramente más baja que la temperatura del resto del cuerpo. La mayoría de los pacientes con lepra subpolar (BL) tuvieron dedos mucho más fríos y lentas velocidades de flujo; los pacientes tuberculoides supolares (BT) tuvieron temperaturas dérmicas similares a las encontradas en los sujetos sanos pero sus velocidades de flujo sanguíneo estuvieron reducidas. La ocurrencia de dedos fríos y baja velocidad de flujo, estuvo claramente asociada con disminución sensorial al toque ligero, a la presión y a la temperatura. Las velocidades de flujo sanguíneo más lentas en las puntas de los dedos estuvieron fuertemente asociadas con alteración del control vasomotor en esta región anatómica, sugiriendo que ambas alteraciones podrían ser una consecuencia de la neuropatía leprosa periférica en los pacientes con lepra temprana, aunque es posible que la arteriopatía leprosa también pueda contribuir a la abatida perfusión periférica en los casos avanzados. Se sugiere que el simple signo clínico de dedos fríos puede ser de valoren la clasificación preliminar de los pacientes que se presentan en cualquier clínica de control de la lepra en las regiones tropicales.During the course of an investigation in India into the impairment of vasomotor reflex control of fingertip blood How as a possible early indicator of peripheral neuropathy in leprosy (5), it was noted that many of the leprosy patients had very cold hands even though the ambient temperature was 26ºC-29ºC. By contrast, healthy Indian control subjects had warm hands under the same environmental conditions. This clinical finding does not seem to have been studied in detail previously, and this paper describes the results of our investigations into the relationship between cold fingers, blood flow in the fingertip skin, and sensation in the fingers.
MATERIALS AND METHODS
Patients. Twenty-two long-standing leprosy patients, all currently receiving standard WHO-recommended multidrug therapy (MDT), were admitted to the study: 13 were inpatients at the Richardson Leprosy Hospital, Miraj, India, for treatment of leg/ foot ulcers and deformity and 9 were attending the outpatient clinic. The disease was classified as borderline lepromatous (BL) in 16 (mean age 35.6 years, S.D. 12.3) and as borderline tuberculoid (BT) in 6 patients (mean age 31.2 years, S.D. 14.0) (25). None of these patients had leprosy skin lesions on their hands, and there was no obvious reabsorption of their fingers.
Controls. Sixteen subjects (mean age 29.9 years, S.D. 6.7) were recruited in Miraj. This group consisted of 11 apparently healthy Indian leprosy control workers and live apparently healthy European subjects who were working at or visiting the Richardson Leprosy Hospital at the time of the study. All participants were volunteers and the study had been approved by the local Ethics Committee.
All subjects were allowed to equilibrate at an ambient temperature of 26ºC-29ºC (the daytime shade temperature at Miraj) while sitting comfortably for 15 min. The tests were always performed in the following sequence: first, assessment of sensation, then skin temperature recording and, lastly, skin blood How measurement. At least eight fingers were examined in all subjects.
Sensory testing. Light touch was assessed first, then pressure sensation and, finally, temperature discrimination. For all tests the subject was seated comfortably with the forearms resting on a table at heart level, the eyes closed, and the head turned away from the side being tested. Care was taken to ensure that subjects-especially nonEnglish-speaking Indian subjects-understood fully the nature of the tests at the beginning of each session. The intervals between tests and the order of testing of the fingers were deliberately varied so that a pattern would not be predicted by the patient.
Light touch was tested by the operator dabbing the skin of the fingers and fingertips lightly with cotton wool. When the subject felt the touch, he/she acknowledged sensation by pointing to the site with the index finger of the contralateral hand. At least 20 sites were tested on each subject's hands, including the palmar and dorsal aspects of all fingers.
Pressure sensation was assessed in a similar manner by use of standard, 6.1 mm, nylon von Frey hairs, one inch long. The hairs bend at a critical pressure, enabling a standard pressure stimulation to be applied (19)
Temperature sensation was assessed with a Thermal Sensibility Sensor (26). Both ends have identical round metal discs: one can be heated to 45ºC with an internal dry battery while the other remains unheated and feels cold by comparison. The subject was asked to indicate whether each application was hot or cold.
The results of all sensation tests on each aspect of a finger were recorded on a threepoint scale: 1 = absent/consistently mistaken; 2 = partial sensation (i.e., variation from area to area on the volar or dorsal aspect of the linger of positive and negative results); 3 = unimpaired sensation. For convenience of analysis the final summary of results on each subject was the mean score (out of 3) for each of the three types of sensation tested, giving a maximum total sensory score of 9.
Measurement of skin temperature. A platinum skin thermistor attached to a LCD output device was used to measure skin surface temperature. The probe (Model 4098, 9 mm diameter; Yellow Springs Instrument Co. Inc., Yellow Springs, Ohio, U.S.A.) was held in close contact with the skin with a single strip of Millipore adhesive tape. The apparatus had been calibrated against an infrared bolometer (Model KT-41; Heimann GmBH, Wiesbaden, Germany). A stable temperature was generally achieved after 5 min of contact with the skin of the pulp of the fingertip or the chest wall. The same sensor was used to measure atmospheric temperature when fully shaded from sunlight.
Measurement of skin blood flow. A laser Doppler velocimeter (PF2; Perimed, Stockholm, Sweden) was used with settings "gain = 3, 12 kHz bandwidth, 0.2 sec time constant and artefact filter off." A probe holder was attached to the pulp of the fingertip with double-sided adhesive tape (Perimed). This ensured that the probe head was located close to, and orientated perpendicular to. the skin surface. The principles of measurement of cutaneous blood flow by laser Doppler methods have been described previously (5,21,22). In brief, an optical fiber directs light from a 2-mW helium-neon laser into the skin. The reflected light from the tissue is collected by other optical fibers for measurement of its intensity and wavelength shift. The relative intensity of the Doppler-shifted component of the reflected light (termed RBC flux and measured in volts) is related to the velocity of movement and the number of moving erythrocytes in the dermal blood vessels under the probe. This machine is internally standardized and it has proved reliable in clinical investigations (3, 6).
Statistical analysis of results. Where appropriate, / tests, ANOVA and discriminant analyses were performed on a microcomputer with a commercial package (Statgraphics, version 3.0; STSC, Inc., Rockville, Maryland, U.S.A.).
RESULTS
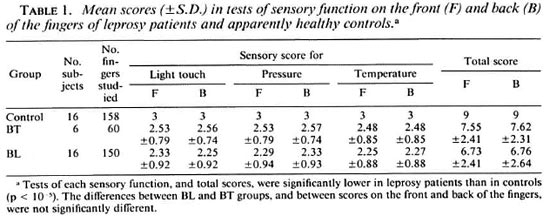
Sensory testing. The control subjects did not show impairment of any of the three sensations tested. Both borderline lepromatous (BL) and borderline tuberculoid (BT) patients' results were significantly different from the controls for each sensation and the total sensory score. Temperature sensation appeared to be the most severely affected in both BL and BT patients but the differences between these two groups in the impairment of this and the other two sensations were not statistically significant (p < 0.08). In the leprosy patients, the little linger showed a small, but significantly (p < 0.05). greater impairment of all three sensations than the thumb. The results are summarized in Table 1.
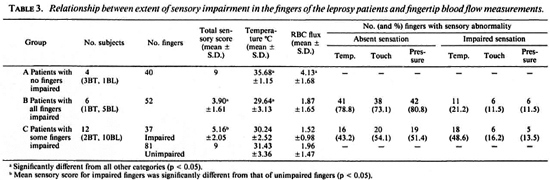
Fingertip skin temperature. There was no significant difference between the control subjects (mean + S.D. = 33.99 ± 2.19ºC) and the BT patients (34.91 ± 1.53ºC), but the BL patients had colder fingers (30.26 ± 3.23ºC) (p < 0.05). Although in many patients some fingers were colder than others, there was no selective involvement of any digit.
Fingertip blood-flow velocity. The RBC flux was high in the control subjects who were showing maximal peripheral vasodilatation AT the ambient temperature of 26ºC29ºC (5.28 ± 1.80 V). By contrast, the BT patients had lower fingertip RBC fluxes (3.62 ± 1.59 V) and the BL patients had even lower blood-flow velocities (1.74 ± 1.49 V). Analysis of variance showed that the three groups were SIGNIFICANTLY different from EACH other (p < 0.05). There were no significant differences in the blood-flow velocity among the individual digits in subjects in any of the groups.
Relationship between skin temperature and RBC flux. The mean values for the measurements in the hands of each patient arc shown in The Figure. With one exception, the control subjects had warm fingers and high RBC fluxes; no reason was found for this subject differing from the other control subjects. In general, the BL patienls had very cold fingers and low RBC fluxes, while the small number of BT patients occupied an intermediate position.
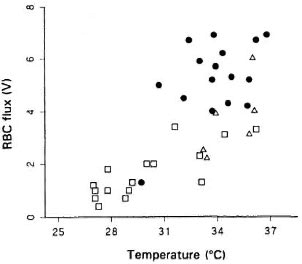
The figure. Relationship between fingertip skin temperature and blood flow( RBC flux) in healthy controls(●), BR leprosy patients (Δ), and BL leprosy patients(□).
Table 2 summarizes the results of discriminant analysis of mean values of all the measurements on the fingers of the individual subjects for temperature and RBC flux. The combination of these two measurements is of potential value in discriminating between the three groups -controls, BL and BT leprosy patients. Additional analysis of the results of the individual fingers confirmed the clear discrimination among the groups, but did not improve the separation significantly.
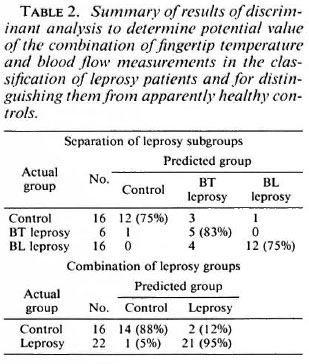
Table 2 also summarizes a subsequent discriminant analysis on the pairs of observations to determine the extent of separation of healthy subjects from leprosy patients of either clinical type. There appears to be considerable diagnostic power in this combination of observations.
Relationship between sensory function and fingertip temperature and blood-flow measurements in leprosy patients. The patients without evidence of sensory impairment in any fingers (Group A, 3 BT and 1 BL) had high temperature and blood flow in their fingertips. Those with sensory impairment in all of their fingers (Group B, 1 BT and 5 BL) had significantly colder fingers and lower RBC flux than those in Group A (p < 0.05). The patients with sensory impairment in some, but not all, fingers (Group C, 2 BT and 10 BL) did not show statistically significant differences in cither skin temperature or blood flow between the affected and unaffected fingers (Groups CI and C2), but both temperature and RBC flux were significantly lower than those found in Group A (p < 0.05). Table 3 summarizes the results.
DISCUSSION
The skin of the fingertip has a major thermoregulatory role, and reflex adjustments of skin blood flow can have a large efTect on body heat loss (16). Any tendency in healthy subjects toward elevation of core temperature (e.g., at high ambient temperature) promotes relaxation of sympathetic vasoconstrictor tone of the arteriovenous anastomoses in the deep dermis, inducing high blood flow in the fingertips (7, 14). The temperature of fingertip skin is strongly influenced by the opening and closing of these vessels although the relationship between skin surface temperature and the underlying blood flow is complex (29), especially when blood flow is estimated noninvasivcly by videophotometric methods (23,27) or by laser Doppler flowmetry (20).
With a single exception, the healthy subjects in this investigation had skin temperatures greater than 31 ºC and high blood flow rates (4.0 V-7.0 V) at an ambient temperature of 26ºC-29ºC. These values were very similar to those obtained by laser Doppler flowmetry on healthy European subjects with centrally induced maximal vasodilatation (l7). The exception was an apparently healthy leprosy control worker; no clinical explanation was found for the failure to demonstrate the usual heat adaptation response, but we have shown that healthy individuals frequently exposed to patients with active leprosy have a higher prevalence of impairment of the cold-induced vasomotor reflex than the unexposed population (1).
All six 13T patients had fingertip skin temperatures appropriate to their environment (greater than 31ºC), but live of these patients showed lower blood-flow velocities than the control subjects. Eleven of the BL patients had skin temperatures less than 31ºC and all had slow fingertip blood flow; the remaining five BL patients had skin temperatures appropriate to their environment but their blood flow was low (1.5 V to 3.5 V). The leprosy patients reported in this study were all Indians, but we (Crée, Abbot, Larshmipati and Beck, unpublished observations) have recently shown that a Caucasian patient with long-standing BL leprosy living in Scotland had substantial reduction in fingertip blood flow when equilibrated in an environment-controlled room with an air temperature of 27ºC. Accordingly, the phenomenon of cold lingers/ low blood flow in leprosy patients is likely to be related to their disease and not to their race or living conditions.
There is strong histopathological evidence that, in some patients, leprosy can cause substantial damage to the larger blood vessels(8, 11, 13) and artériographie studies have confirmed that there are important functional changes, such as impairment of blood flow in the terminal vascular loops (24), narrowing and constriction of the arteries of the lower limb (9, 12), and tapering tortuosity of the arteries of the hands (2,10, 30). Venous involvement has also been reported (4, 18). These changes are most severe in patients with obvious clinical involvement of the fingers, especially progressing reabsorption, but they can be detected in some patients without clinical evidence of arterial disease or noticeable physical deformity (28).
The occurrence of slow blood flow and cold fingers could, therefore, be the result of an arteriopathy but since these features are strongly related to sensory impairment in individual leprosy patients, it is likely that such patients have substantial peripheral neuropathy. In a previous study of a large group of leprosy patients, the lowest fingertip blood-flow measurements were recorded in those with substantial neuropathy and/or orthopedic complications, but low flow was also observed in some newly registered patients with impairment of the vasomotor autonomic reflexes (1). It was also found that some patients with early leprosy had focal and selective impairment of vasomotor autonomic reflexes but relatively normal fingertip blood flow. This led us to infer that such impairment of the reflexes was the result of damage to the autonomic conduction pathways in the peripheral nerves rather than to the loss of vascular wall responses due to arteriopathy. Nevertheless, there is little doubt that arteriopathy plays a major role in the pathogenesis of cold fingers in patients with advanced disease. It is also possible that denervation hypersensitivity to catecholamines contributes to the peripheral circulatory abnormalities in leprosy patients.
Although cold fingers in environmental conditions requiring maximal vasodilatation for thermoregulation do not always occur in leprosy, the clinical sign is sufficiently frequent in the disease to suggest that there may be considerable value in feeling the patient's hands as a preliminary to clinical examination in any leprosy control clinic in the tropics.
Acknowledgment. LEPRA generously supported NCA and JSB for their visit to Miraj, India. We are grateful to the stalf nurses at the Richardson Leprosy Hospital, Miraj, Messrs. C. Bhosale, N. Bhopale and M. Sagare for valuable assistance in the clinical work, to Dr. J. H. Gibbs for help with the statistical analysis and preparation of the figure, and to Mrs. R. Mitchell and Mrs. S. Neil for valuable secretarial assistance.
REFERENCES
1. ABBOT, N. C, BECK, J. S., SAMSON, P. D., BROWN, R. A., GRANGE, J. M., CRÉE, I. A. and BUTLIN, C. R. Impairment of fingertip vasomotor reflexes in leprosy patients and apparently healthy contacts. Int. J. Lepr. 59(1991)537-547.
2. AGRAWAL. B. R. and AGRAWAL, R. I. Arteriography in leprosy. Indian J. Lepr. 57(1985)138-145.
3. AGUSNI, I., BECK, J. S., POTTS, R. C, CRÉE, I. A. and ILIAS. M. I. Blood flow velocity in cutaneous lesions in leprosy. Int. J. Lepr. 56(1988)394-400.
4. BANSAL, R.. KAUR, B., SHARMA, V. K., KATARIYA, S., CHAKRAVARTI, R. N. and BUSHARNAMATH, S. R. Venous involvement in leprosy: a venographic and histopathologic correlation. Int. J. Lepr. 55(1987)499-506.
5. BECK, J. S.. ABBOT, N. C, SAMSON, P. D., BUTLIN, C. R., GRANGE, J. M.. CRÉE, I. A., FORSTER, A. and KUAN, F. Impairment of vasomotor reflexes in the fingertips of leprosy patients. J. Neurol. Neurosurg. Psychiat. 54(1991)965-971.
6. BECK, J. S. and SPENCE, V. A. Patterns of blood flow in the microcirculation of the skin during the course of the tuberculin reaction in normal human subjects. Immunology 58(1986)209-215.
7. BINI, G., HAGBARTH, K. E.. HYNNINEN, P. and WALLIN, P. G. Thermoregulatory and rhythm generating mechanisms governing sudomotor and vasoconstrictor outflow in human peripheral nerves. J. Physiol. 306(1980)537-552.
8. CARAYON, A. Investigations on the physiopathology of the nerve in leprosy. Int. J. Lepr. 39(1985)278-294.
9. CHANDRAHASON-JOHNSON, A., REDDY, R., JOHNSON, S. and EVERETTE-JAMES, A. Lower limb angiography in leprosy. Radiology 126(1978)327-332.
10. CHOPRA, J. S., KAUR, S., MURTHY, J. M., KUMAR, B., RADIIAKRISHNAN, V., SURI, S. and SAWHNEY, B. B. Vascular changes in leprosy and its role in the pathogenesis of leprous neuritis. Lepr. India 53(1992)443-453.
11. CORUH, G. and MCDOUGALL, A. C. Untreated lepromatous leprosy - histopathological findings in cutaneous blood vessels. Int. J. Lepr. 47(1979)500-511.
12. DEBÍ, B. P., MOHANTY, II. CTRIPATHY, N.,TOMPE, D. B. and SARANGI, B. K. Artériographie pattern of plantar ulcers in lepromatous leprosy. Lepr. India 52(1979)429-432.
13. FITE, G. L. The vascular lesions in leprosy. Int. J. Lepr. 9(1941)193-202.
14. HALES, J. R. S., JESSON, C, FAUCETT, A. A. and KING, R. B. Skin AVA and capillary dilatation and constriction induced by local skin heating. Pllugcrs Arch. 404(1985)203-207.
15. HOLLOW AY, G. A. Laser Doppler measurements of cutaneous blood How. In: Non-Invasive Physiological Measurement, Vol. 3. Rolfe, P., ed. London: Academic Press, 1983, pp. 219-249. 26.
16. JOHNSON, J. M., BREGGELMANN, G. L., HALES, J. R. S., VAN HOUTTE, P. M. and WENGER, C. B. Regulation of the cutaneous circulation. Fed. Proc. 45(1986)2841-2850. 27.
17. KHAN, F., SPENCE, V. A., WILSON, S. B. and ABBOT, N. C. Quantification of sympathetic vascular responses in skin by laser Doppler llowmetry. Int. J. Microcirc. Clin. Exp. 10(1991)145-153.
18. MUKHERJEE, A., GIRDHAR, B. K., MALVIYA, G. N., RAMU, G. and DESIKAN, K. V. Involvement of subcutaneous veins in lepromatous leprosy. Int. J. Lepr. 51(1983)1-6.
19. NAAFS, B. and DAGNE. T. Sensory testing; a sen29.sitive method in the follow-up of nerve involvement. Int. J. Lepr. 45(1977)364-368.
20. NEUFELD, G. R., REILLY, C. A., GALANTE, S. R., ROBERTS, A. B., BAUMGARDNER, J. E., GRAVES, D. J. and QUINN, J. A. Response of cutaneous laser velicometry to temperature change: normals and dysvascular patients compared. Vase. Surg. 21(1987)331-338.
21. NILSSON, G. E., TENLAND. T. and OBERG, P. A. A new instrument for continuous measurement of tissue blood How by light beating spectroscopy. IIEE Trans. Biomed. Eng. 27(1980)12-19.
22. NILSSON, G. E., TENLAND. T. and OBERG, P. A. Evaluation of a laser Doppler flowmeter for measurement of tissue blood How. IIEE Trans. Biomed. Eng. 27(1980)597-604.
23. OSTERGREN, J. and FAGRELL, E. Skin capillary blood cell velocity in man; characteristics and reproducibility of the reactive hyperaemia response. Int. J. Microcirc. Clin. Exp. 5(1986)37-51.
24. PATERSON, D. E. Radiological bone changes and angiographic findings in leprosy: with special reference to the pathogenesis of'atrophic' conditions of the digits. J. Fac. Radiol. 6(1989)35-56.
25. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
26. SRINIVASAN, H. and STUMPE, B. Value of thermal sensibility testing in leprosy diagnosis in the field - field trial of a pocket device. Lep. Rev. 60(1989)317-326.
27. TOOKE. J. E., OSTERGREN, J. and FAGRELL, E. Synchronous assessment of human skin microcirculation by laser Doppler llowmetry and dynamic capillaroscopy. Int. J. Microcire. Clin. Exp. 2(1983)277-284.
28. WAHI, P. L., KAUR, S., VADWA, M. B., SODHI, J. S. and CHAKRAVATI, R. N. Peripheral artériographie studies in leprosy. Clin. Radiol. 27(1976)365-370.
29. WILSON. S. B. and SPENCE, V. A. A tissue heat transfer model for relating dynamic skin temperature changes to physiological parameters. Phys. Biol. Med. 33(1988)895-912.
30. YADAV, S. S. Artériographie evaluation of vascular changes in leprosy. Angiology 29( 1978)17-21.
1. M.Sc; Research Assistant, Department of Pathology, University of Dundee, Dundee, U.K.
2. M.D., F.R.C.Path.. F.R.S.E.; Professor, Department of Pathology, University of Dundee, Dundee, U.K.
3. M.B.B.S., M.P.H.; Superintendent; Medical Student, Richardson Leprosy Hospital, Miraj, Dist. Sangli. Maharashtra, India.
4. Medical Student, Richardson Leprosy Hospital, Miraj, Dist. Sangli. Maharashtra, India.
5. M.B., B.Ch.; Medical Officer, Anandaban Hospital, Kathmandu, Nepal.
6. M.D., M.Sc; Reader, Department of Microbiology, National Heart and Lung Institute, Royal Brompton Hospital, Sydney Street, London SW3 6NP, U.K.
Reprint requests to Dr. Grange.
Received for publication on 24 April 1992.
Accepted for publication in revised form on 28 July 1992.