- Volume 60 , Number 4
- Page: 525–35
Relapses in multibacillary leprosy patients atter stopping treatment with rifampin-containing combined regimens
ABSTRACT
During the decade between the mid 1970s and the mid-1980s, 12 rifampin (RMP)-containing combined regimens were tested among lepromatous leprosy patients in the Institut Marchoux. The 384 patients who were seen at least once during the period beginning 12 months after completion of treatment were considered eligible for analysis of the relapse rate. By the end of May 1991, relapse, manifested by a significant increase of the bacterial index (BI) and the appearance of new lesions with a BI greater than that of preexisting lesions, had been observed in 68 (17.7%) of these patients. Relapse was confirmed by the presence of viable Mycobacterium leprae in skin biopsy specimens obtained f rom 54 of the first 61 cases; virtually all of the isolated strains remained susceptible to RMP. The relapses occurred late, about 5 ± 2 years after stopping treatment; the shorter the duration of RMP administration, the earlier the appearance of the relapse. The variations of the relapse rate among regimens were considerable: total relapse rate ranged f rom 2.9% to 27.8%, and the relapse rate per 100 patient-years of observation ranged f rom 0.8 to 6.9. Among the 12 regimens, only the WHO/MDT yielded an acceptable relapse rate (defined as a rate lower than 1.0 per 100 patient-years). However, because the mean duration of follow up was shortest among the patients treated with WHO/ MDT, the relative low relapse rate among these patients must be interpreted with great caution.RÉSUMÉ
Au cours de la décennie s'étendant du milieu des années 1970 au milieu des années 1980, 12 regimes combines comprenant de la rifampicinc (RMP) on été testes à l'Institut Marchoux chez des patients présentant une lèpre lépromateuse. Les 384 patients qui ont été vus au moins une Ibis au cours de la période commençant 12 mois après la lin du traitement ont été considérés comme eligióles pour l'analyse du taux de rechute. Fin mai 1991, une rechute, se manifestant par une augmentation significative de l'indice bactérien (IB) et l'apparition de nouvelles lésions avec un IB plus élevé que celui observé au niveau des lésions pré-existantes, avait été observée chez 68 (17.7%) de ces patients. La rechute était confirmée par la présence de Mycobacterium leprae viables dans des specimens de biopsie cutanée obtenus chez 54 des 61 premiers cas; pratiquement toutes les souches isolées restaient susceptibles à la RMP. Les rechutes apparaissaient tardivement, environ 5 ± 2 ans après l'arrêt du traitement; au plus courte était l'administration de RMP, au plus précoce était la réchute. Les variations du taux de rechute entre les dillérents régimes étaient considérables: le taux total de rechute variait de 2.9% à 27.8%, et le taux de rechute calculé pour 100 pesonnesannées d'observation variait de 0.8 à 6.9. Parmi les 12 régimes, seule la PCT telle que recommandée par l'OMS s'accompagnait d'un taux de rechute acceptable (défini comme un taux inférieur à 1.0 pour 100 personnesannées). Cependant, du fait que la durée moyenne du suivi était la plus courte parmi les patients traités par la PCT/OMS, le taux de rechute relativement bas observé chez, ces patients doit être interprété avec une grande prudence.RESUMEN
Entre mediados de los 1970 y mediados de los 1980. se probaron 12 esquemas de tratamiento combinado conteniendo rifampina (RMP). en los pacientes con lepra lepromatosa del Instituto Marchoux. Trescientos ochenta y cuatro pacientes que completaron el tratamiento fueron seguidos para establecer su frecuencia de recaída. A finales de mayo de 1991, 68 pacientes (17.7%) habían ya presentado recaídas. Las recaídas se manifestaron como un ircremento significativo en el índice bacteriano (IB) y como aparición de nuevas lesiones con IB mayores que los de las lesiones preexistentes. Las recaídas también se confirmaron por las presencia de Mycobacterium leprae viables en las biópsias de piel obtenidas de 54 de los primeros 61 casos; prácticamente todas las cepas aisladas permanecieron susceptibles a la RMP. Las recaídas ocurrieron tarde, después de 5 ± 2 años de suspender el tratamiento; mientras más corta fue la duración del tratamiento, más temprana fue la aparición de la recaída. Las variaciones en la frecuencia de recaídas entre los diferentes esquemas fueron considerables: la frecuencia total de recaídas osciló entre 2.9 y 27.8%, y el grado de recaídas por 100 paciente-años de observación estuvo entre 0.8 y 6.9. Entre los 12 esquemas de tratamiento, sólo la poliquimioterapia recomendada por la OMS condujo a un grado aceptable de recaídas (menor de 1.0 por 100 paciente-años). Sin embargo, debido a que la duración media de seguimiento fue más corta en los pacientes tratados con el esquema de la OMS, la relativamente baja frecuencia de recaídas en estos pacientes debe interpretarse con gran cautela.Until multidrug therapy (MDT) was recommended by a WHO Study Group in 1981 (20), dapsone (DDS) administration as monotherapy, often of life-long duration, was the standard treatment of individual leprosy patients, and the standard regimenfor leprosy control programs throughout theworld. A large proportion of patients withmultibacillary (MB) leprosy sustained re-lapse during DDS monotherapy (1, 10, 13, 19), many of whom demonstrated Mycobacterium leprae resistant to DDS (7). Because the relapse rate among patients whose treatment was stopped after 20 years of daily supervised treatment with DDS was found to be 1 per 100 patient-years of observation (19), it appeared that ensuring the regularity of DDS monotherapy over the long term did not substantially change the picture. Therefore, it was clear that attempts to control leprosy by DDS monotherapy were failing, and that it was necessary to replace this treatment by the administration of a combination of drugs, a method that had proved successful in preventing the relapse of tuberculosis and the selection of drug-resistant mutants of M. tuberculosis (11). However, unless the combined treatment was effective if administered for only a limited period of time, and led to a more rapid "cure," it would be unlikely to increase the effectiveness of leprosy control activities.
Because the very powerful and rapid bactericidal activity of rifampin (RMP) against M. leprae had been demonstrated in earlier studies (2, 15), various short-term RMP-containing combined regimens were teste damong MB patients at the Institut Marchoux, Bamako, Mali, during the decade beginning in the mid-1970s. Despite many difficulties, a majority of the patients were followed up after stopping treatment, providing a unique opportunity to assess the effectiveness of different RMP-containing combined regimens in terms of relapse rates. A great majority of the relapses described in the present communication have been confirmed by mouse foot pad inoculation, a method rarely employed in studies of relapse of MB leprosy (3, 5, 19).
MATERIALS AND METHODS
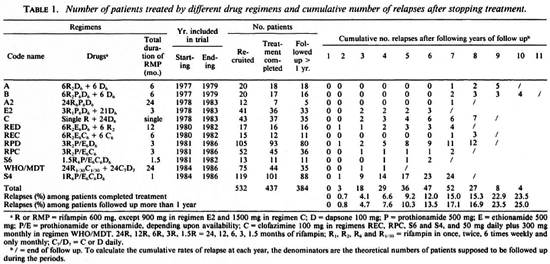
Regimens. Between 1977 and 1986, 12 RMP-containing combined regimens, as shown in Table 1, were tested. The total duration of treatment ranged from 1 to 24 months, and the duration of RMP administration ranged from a single dose to daily doses for 24 months. The administration of all the drugs, even those administered daily, was supervised. No subsequent antileprosy therapy was prescribed to patients after the trials.
Patients. A total of 532 MB patients were recruited into the trials. All of them demonstrated active leprosy skin lesions with a bacterial index (BI) of > 2+ and a negative Mitsuda lepromin reaction (< 3 mm in diameter). Histopathological examination had been conducted in 382 (72%) patients, and the classification of lepromatous (LL) or borderline lepromatous (BL) leprosy had been confirmed. The patients were hospitalized during the trials until completion of chemotherapy. All patients treated with regimens A, B, A2, E2, C, and S6 had not been treated previously. In patients treated with other regimens, 55.9% (214/383) had not been treated previously, 37.1% (142/383) had been treated with various durations of DDS monotherapy, and 7.0% (27/383) had been treated with various RMP-containing regimens, mostly single-dose RMP 1500 mg plus daily DDS. Regimens REC and RPC were administered to patients who had been treated previously with DDS monotherapy for longer than 5 years, or who had relapsed with DDS-resistant M. leprae proven by mouse foot pad inoculation.
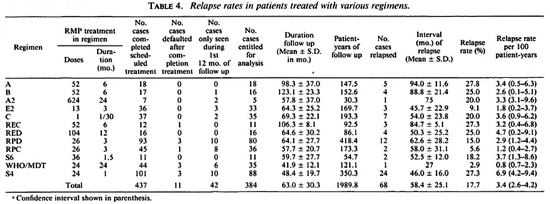
Of the 532 patients, 437 completed the scheduled treatments. Among the latter, 53 are not suitable for analysis of relapse: 11 had never returned for follow-up examination by the end of May 1991 ; and 42 had been seen only during the first 12 months after completing treatment (Table 4). Thus, among the 437 eligible patients, only 384 cases have been seen at least once later than 12 months after completing treatment, and may be used for calculating the relapse rate (Table 4). Of these 384 cases, 255 cases had not received antileprosy treatment previously, 46 cases had been given DDS monotherapy for less than 5 years, 22 with singledose RMP 1500 mg plus daily DDS, 61 with DDS monotherapy for at least 5 years, and 12 of the latter group had relapsed with proven DDS-resistant M. leprae.
Procedures of follow up. At the time of each patient's visit after treatment had been completed, a thorough clinical examination was carried out with special emphasis on the evolution of preexisting leprosy lesions and detection of suspected new lesions; skin smears were taken from the six sites originally examined, and from any suspected new lesion(s); and a urine specimen was collected for DDS analysis. The slid cs of the skin smears were stored in the dark, at least until the results of the next examination became available.
Criteria of relapse. Relapse was suspected when the BI at any site was found to have increased by at least 2+ over the previous value and the BI increase was confirmed by reexamination; or when a definite new lesion was observed with a BI greater than that in any preexisting lesion. In most cases, a skin biopsy was taken from the suspected new lesion for enumeration of M. leprae, expressed as the log10 number per gram of tissue (LOGAFB), mouse foot pad inoculation to test the viability (l7) and drug susceptibility (8) (mainly to RMP and DDS) of the organisms, and histopathological examination. The first two items were examined mainly at the Institut Marchoux; some of the specimens were examined in the laboratory of S. R. Pattyn (Antwerp) or of J. Grosset (Paris). Detection of viable organisms, defined as multiplication to > 105 organisms per foot pad after inoculation of 5 × 103 organisms per foot pad of immunocompetent mice, was taken as confirmation of the relapse. The histopathological examinations were carried out in the laboratory of S. R. Pattyn or that of G. Discamps (Bordeaux). Histopathological relapse was defined as the appearance of young histiocytic granulomas containing solidly stained bacilli either isolated or in globi.
Statistical analysis. The relapse rates among groups of patients treated with various regimens were compared by Fisher's exact probability test. The "incubation period" of relapse after completion of treatment was calculated as the number of months between stopping treatment and the appearance of the relapse, and the latter was defined as the midpoint between the dates of the last examination without relapse and the first examination revealing evidence of relapse. The mean values were compared by Student's t test.
RESULTS
Follow up and relapses. Among the 384 cases followed up more than 1 year, the mean duration of follow up was 63.0 ± 30.3 months after completion of treatment (Tables 1 and 4). It varied widely among patients treated with different regimens, ranging from 41.9 ±12.1 months among patients treated with WHO/MDT to 123.1 ± 23.3 months among patients with Regimen B. Urine analysis for DDS was negative for more than 90% of the examinations, suggesting that the great majority of these patients had indeed stopped chemotherapy during the period of follow up.
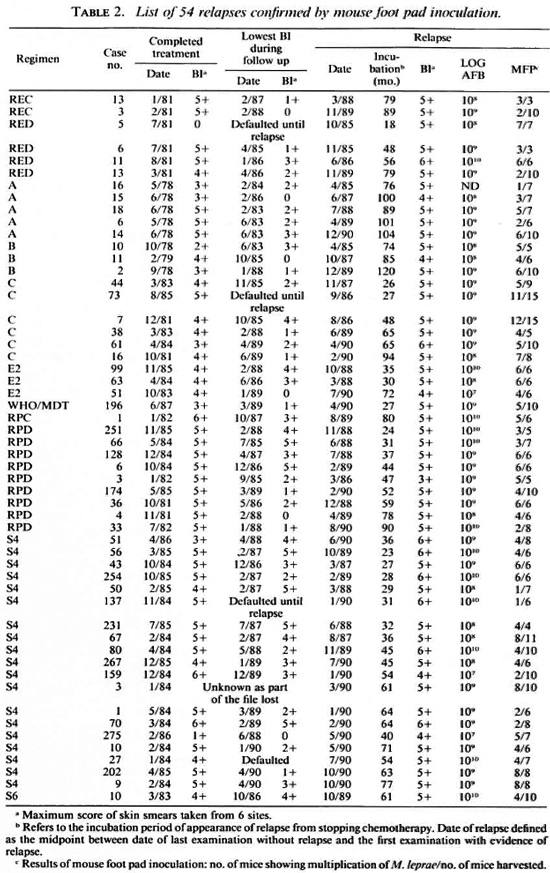
After stopping chemotherapy, the majority of patients showed continuing clinical improvement as well as a declining BI, regardless of the regimens they had received (Tables 2 and 3). In fact, 113 cases (nearly 30% of those followed up) reached smear negativity during the follow-up period. However, by the end ofMay 1991 (as shown in Tables 2 and 3) new lesions, mainly nodules and/or lepromas, together with high bacterial loads in terms ofBI and LOGAFB, had occurred in 68 cases, suggesting that these patients had relapsed. In the histopathological examinations of these 68 patients, relapse was highly suggested in 27 of them by the appearance ofyoung histiocytic granulomas containing numerous solidstaining bacilli either isolated or in globi.
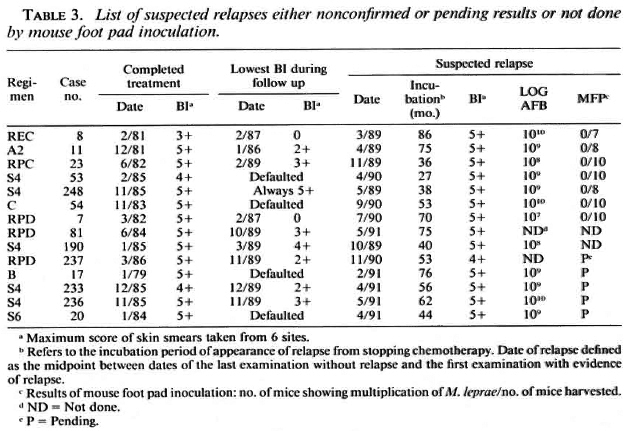
As shown in Table 2, viable organisms were detected and, therefore, relapse was confirmed in 54 (88.5%) of the first 61 cases of suspected relapse for which the results of mouse foot pad inoculation are available. Although, as shown in Table 3, the organisms from the remaining 7 cases failed to multiply in mice, this proportion of cases in which multiplication could not be demonstrated is similar to that observed among previously untreated cases (6, l2, l7). Therefore, failure of the organisms to multiply in mice does not exclude relapse in these cases. Of the additional 7 patients suspected of having relapsed, the results of mouse inoculation from 5 cases are still pending, and mice were not inoculated with organisms from 2 cases when relapses were suspected because of a lack of animals. Because, as is also shown in Table 3, the clinical and histopathological manifestations and the numbers of organisms did not differ significantly from the corresponding features of the 54 cases whose relapses were confirmed (see Table 2), relapses of the 7 cases should also be diagnosed. In brief, all 68 suspected relapses may be taken to have been confirmed.
Relapse rates among patients treated with various regimens. As shown in Tables 1 and 4, the overall relapse rate among 384 patients was 17.7%, ranging from 2.9% among the patients treated with WHO/MDT to 27.8% among those treated with regimen A, and was no less than 15% among the patients treated with 9 of the 12 regimens. Although the relapse rate among patients treated with WHO/MDT did not differ significantly from those among the patients treated with Regimens A2, E2, S6 and RPC (p > 0.05), it was significantly less than those of patients treated with Regimens A, B, C, REC, RED. RPD and S4 (p < 0.05 or < 0.01).
The relapse rate per 100 patient-years of observation for all 384 patients was 3.4, ranging from 0.8 for WHO/MDT to 6.9 for Regimen S4. The rate in patients treated with the WHO/MDT was significantly less than that in patients treated with Regimen S4, but did not differ significantly from the relapse rates among the patients treated with any of the other regimens.
No correlation was observed between the relapse rate and the duration of RMP administration. As shown in Table 1, 50 (17.7%) of the 283 patients treated with RMP for 3 months or less relapsed; whereas 18 (17.8%) of the 101 patients treated with RMP for longer than 3 months relapsed.
Incubation period of relapse. No relapse occurred during the first year after stopping treatment among the 384 patients followed up more than 1 year and the 42 patients seen only during the first 12 months after completion of treatment. Among the 68 patients whose relapses were confirmed, the mean interval between stopping treatment and the appearance of the relapse, i.e., the incubation period of relapse, was 58.4 ± 25.1 months, ranging from 27 months in the patient treated with WHO/MDT to 94.0 ± 11.6 months among those treated with Regimen A (Table 4); 32 relapses, nearly 50%, appeared no earlier than 6 years after stopping treatment. The overall cumulative percentage of relapse appears to increase regularly by about 3% per year from year 3 to year 10 of follow up. On the other hand, the shorter the duration of RMP administration, the shorter the incubation period of relapse; the incubation period was < 5 years for 32 (64%) of the 50 relapses among patients treated with RMP for < 3 months; whereas only 4 (22.2%) of the 18 relapses among patients treated with RMP for > 3 months appeared within 5 years of stopping treatment (p < 0.01). In view of the correlations between the length of follow up and the risk of relapse, and between the length of treatment and the incubation time of relapse, the smaller relapse rate among the patients treated with WHO/MDT should be considered as only provisional.
BI upon completion of treatment and risk of subsequent relapse. Among the 197 patients whose maximal BI was >5 + upon stopping treatment, relapses occurred in 42 (21.3%). Relapses occurred in only 25 (13.6%) of the 184 cases whose maximal BI was < 4 +. The proportions of relapse differ significantly between the two groups (p < 0.05), suggesting that the risk of relapse was greater among the patients with higher bacterial loads at the time of stopping treatment. Of the 384 patients, 113 achieved skin-smear negativity during the follow-up period and relapses appeared in only 8 (7.1%) of these patients; whereas relapses occurred in 60 (22.1 %) of the remaining 271 patients who did not achieve smear negativity. Thus, the risk of relapse was significantly smaller among those who achieved smear negativity (p < 0.01). However, achievement of smear negativity does not guarantee that the patients will not relapse.
Drug susceptibility of organisms recovered from relapsed patients. The susceptibility to RMP of the M. leprae recovered from the skin biopsy specimens obtained from 47 relapsed patients was measured by inoculation of mouse foot pads; 45 strains were inhibited from multiplication by the administration of RMP to the mice in a weekly dosage of 10 mg/kg body weight and, therefore, were susceptible to RMP. The organisms recovered from patient no. 43 of those treated with Regimen S4 and from patient no. 11 of those treated with Regimen RPD multiplied in RMP-treated mice. However, in neither case could resistance to RMP be confirmed by subinoculation: the organisms of no. 43 failed to multiply in subinoculated mice while the organisms of no. 11 multiplied in all 10 subinoculated, untreated control mice but in none of the 12 subinoculated mice treated with RMP. Therefore, it is difficult to conclude that these two relapses were associated with the emergence of RMP-resistant M. leprae.
The susceptibility to DDS of 34 of the 47 strains was also tested and 6 (17.7%) strains were found resistant: 3 of low degree, 2 of moderate degree, and 1 of high degree. One of the six strains had been shown to be resistant to DDS before treatment with Regimen RPC. Because primary resistance to DDS among previously untreated MB patients in Mali has been well documented(18 ), it is likely that the remaining four strains were also resistant to DDS before starting the combined therapy.
DISCUSSION
As in the case of other infectious diseases, relapse after cessation of specific antimicrobial treatment is a crucial parameter in assessing the long-term efficacy of chemotherapy in leprosy. Thus far, very limited information is available with respect to the risk of relapse after treatment with RMPmonotherapy (5) and RMP-containing combined regimens (3). Very few studies have involved long-term follow up of patients after stopping treatment with RMP-containing combined regimens (9).
With the introduction of WHO/MDT (20), the treatment of many patients is stopped while skin smears are still positive. As a result, the demonstration of acid-fast bacilli (AFB) in skin smears alone is no longer sufficient for the diagnosis of relapse as it was during the era of DDS monotherapy. Therefore, relapse may now be diagnosed only according to more stringent criteria: a) increase of the BI by > 2 + over the previous value from any single site of old lesions and confirmed on reexamination; b) occurrence of definite new lesion(s) which demonstrate a higher BI than do the preexisting lesions; and (c) demonstration of viable M. leprae, an indicator of bacterial remultiplication, by inoculation of mouse foot pads. However, because the facilities for mouse inoculation are not accessible in many leprosy control programs, and because in the present study viable organisms were demonstrated in nearly 90% of the patients, fulfilling the first two criteria, we believe that these two criteria are sufficient for the diagnosis of relapse under field conditions. In theory, relapse may occur in the absence of obvious new lesion(s) during its early stage but, in our experience, new lesions must occur sooner or later in real relapse. Because the quality of skin smears is far below desirable in many leprosy control programs, if the increase of the BI is marginal and is not accompanied by new lesion(s), we propose that the patient be kept under close surveillance, and the examinations repeated every 3 to 6 months. After all, relapse is not a clinical emergency, and there is no need to make a quick decision.
After the first few doses of treatment with RMP, the M. leprae of virtually all patients lose their infectivity for both immunocompetent mice (2, 15) and immunosuppressed rodents, e.g., thymectomized and irradiated mice (17) and neonatally thymectomized Lewis rats (4). However, one must be careful in interpreting the clinical significance of these findings. Because of the insensitivity of the animal systems, including even the athymic nude mouse, one may measure at best only the initial killing of 5 "logs," i.e., killing of the first 99.999% of the organisms; whereas the untreated lepromatous patients may harbor as many as 1010 viable M. leprae (6). In other words, after his/her organisms have lost infectivity for mice, such a patient may still harbor 105 viable bacilli, still a substantial number. It is not relevant to discuss whether or not these remaining viable M. leprae represent the "persisters" (17) because the key issue is that they are capable of causing relapse, as shown by the results of the present study. Relapse occurred among 17.7% of the patients after stopping treatment with a variety of RMP-containing combined regimens, clearly indicating that they received insufficient treatment.
Another important result of this study is that the relapses occurred late after stopping treatment. As shown in Table 4, the mean incubation period of relapse was about 5 ± 2 years, with the relapses appearing earlier among those receiving the shortest periods of treatment with RMP (Table 1). The late appearance of relapses confirms the assumption that the annual risk of relapse varied according to the length of time since completion of treatment (21). Because, in our study, relapses occurred at about the same time as that recorded after RMP-monotherapy (5), it appears likely that the late appearance is a characteristic of the relapses after treatment with RMP. In addition, because the mean incubation period of the observed relapses was close to the mean duration of follow up in each group (Table 4) it leads to the suspicion that more relapses will occur as the follow up is prolonged, and the mean incubation period of relapse may be longer than that presented in Table 4. Because of the late appearance of relapses, patients should be followed up for at last 7 to 10 years after completing chemotherapy. Only after a prolonged period of follow up may one draw final conclusions with respect to the efficacy of any RMP-containing combined regimen.
The total relapse rate among the patients treated with the 11 regimens other than WHO/MDT was greater than 5%, and was at least 15% among those treated by nine of the regimens. To date, no consensus has been reached with respect to what represents an acceptable relapse rate, and the opinions of clinicians may differ from those of epidemiologists. We believe that, at least in clinical trials, a relapse rate to be acceptable must be lower than 1.0 per 100 patient-years. By this criterion, all of the regimens tested, with the exception of WHO/ MDT, yielded unacceptable rates of relapse. However, the mean duration of follow up of the patients treated with WHO/MDT was the shortest (41.9 ± 12.1 months) among the 12 groups; whereas the mean incubation period of the 68 relapses was 58.4 ± 25.1 months after stopping chemotherapy, and was 94.0 ±11.6 and 88.8 ±21.4 months among patients treated with Regimens A and B respectively. Therefore, the relative low relapse rate among patients treated with WHO/MDT must be interpreted with great caution pending longer follow up. Because millions of leprosy patients have been or currently are being treated with the WHO/MDT regimen, we strongly suggest that the MB patients, especially those whose BI is still high after completion of treatment, be closely followed up.
Because 50% of the patients had been lost to follow up 5 years after completion of treatment, and because of the late appearance of relapse, the observed relapse rate (17.7%) may well represent an underestimate. On the other hand, our results demonstrate that the risk of relapse is greater among those patients whose BI is higher at the time of stopping treatment. Because the great majority of our patients had a high BI, with more than 50% showing a maximal BI > 5+ when stopping treatment, this situation is quite different from that encountered in many control programs, and it may be that the high relapse rates observed in our study will not be observed elsewhere.
Finally, in contrast to the earlier findings that 22 of the 39 strains of M. leprae recovered from patients relapsing after treatment with RMP monotherapy were resistant to RMP (5), almost all of the strains isolated in the current study were demonstrated to be susceptible to RMP. There are two possible explanations. First, the strength of the RMP component in some of the regimens was insufficient to select for the multiplication of RMP-resistant mutants (5), and second, the companion drugs in other regimens prevented the selection of RMP-resistant mutants.
Acknowledgment. This investigation received partial financial support from the UNDP/World Bank/ WH O Special Programme for Research and Training in Tropical Diseases.
REFERENCES
1. CARTEL, J. L., BOUTIN, J. P.. SPIEGEL, A., PLICHART. R. and Roux. J. F. Longitudinal study on relapses of leprosy in Polynesia multibacillary patients on dapsonc monotherapy between 1946 and 1970. Lepr. Rev. 61(1991)186-192.
2. COLLABORATIVE EFFORT OF THE U.S. LEPROSY PANEL (U.S.-JAPAN COLLABORATIVE MEDICAL SCIENCE PROGRAM) and THE LEONARD WOOD MEMORIAL. Rifampin therapy of lepromatous leprosy. Am. J. Trop. Med. Hyg. 24(1975)475-484.
3. CONSTANT-DESPORTES, M., GUELPA-LAURAS, C.-C, CAROLINA, J. C, LEOTURE, A., GROSSET, J.-H. and SANSARRICQ, H. A case of relapse with drug-susceplible M. leprae after multidrug therapy. Int. J. Lepr. 59(1991)475-484.
4. GELBER, R. H., HUMPHRES, R. C. and FIELDSTEEL, A. H. Superiority of the neonatally thymectomized Lewis rat (NTLR) to monitor a clinical trial in lepromatous leprosy of two regimens of rifampin and dapsone. Int. J. Lepr. 54(1986)273-282.
5. GROSSET, J.-H., GUELPA-LAURAS, C.-C, BOBIN, P., BRUCKER, G., CARTEL, J.-L., CONSTANT DESPORTES, M., FLAGEUL, B., FREDERIC, M., GUILLAUME, J.-C. and MILLAN. J. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int. J. Lepr. 57(1989)507-614. 16.
6. GROSSET, J.-H., JI, B., GUELPA-LAURAS, C.-C , PERANI, E. G. and N'DELI, L. N. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatousleprosy. Int. J. Lepr. 58(1990)281-295.
7. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
8. Ji, B. Drug susceptibility testing of Mycobacteri17.um leprae. Int. J. Lepr. 55 Suppl. (1987)830-836.
9. KATOCII, K., NATARAJAN, M., BAGGA. A. and KATOCH, V. M. Clinical and bacteriological prog ress of highly bacillated BL-LL patients discontin uing treatment after different periods of MDT. Int. J. Lepr. 59(1991)248-255.
10. KURZ, X. M., DECLERCQ. E. E. AND VELLUT, C. M. Rate and time distribution of relapses in multibacillary leprosy. Int. J. Lepr. 57(1989599-606.
11. MEDICAL RESEARCH COUNCIL-TUBERCULOSIS CHEMOTHERAPY TRIALS COMMITTEE. Long-term chemotherapy in the treatment of chronic pulmonary tuberculosis with cavitation. Tubercle 43(1962)201-267.
12. N'DELI, L., GUELPA-LAURAS, C.-C, PERANI, E. G. and GROSSET, J.-H. Effectiveness of pefloxacin in the treatment of lepromalous leprosy. Int. J. Lepr. 58(1990)12-18.
13. NOORDEEN, S. K. Relapse in lepromatous leprosy. Lepr. Rev. 42(1971)43-48.
14. PATTYN, S. R., SAINT ANDRE, P., FERACCI. C. and BAQUILLON, G. Comparative study of two regimens of combined chemotherapy of one year duration in multibacillary leprosy; results after four and live years' follow-up. Int. J. Lepr. 52(1984)297-303.
15. SHEPARD, C. C, LEVY, L. and FASAL. P. Rapid bactericidal activity of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
16. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. THELEP controlled clinical trials in lepromatous leprosy. Lepr. Rev. 54(1983)167-176.
17. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
18. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Primary dapsone-resistance in Bamako and Chingleput; final report. Lepr. Rev. 58(1987)209-218.
19. WATERS, M. F. R., REES. R. J. W.. LAING, A. B. G, FAH, K. K., MEADE. T. W., PARIKSHAK, N. and NORTH, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulfone therapy. Lepr. Rev. 57(1986)101-109.
20. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva; World Health Organization, 1982. Tech. Rep. Ser. 675.
21. WHO STUDY GROUP. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
Members of the Study Group are: Leopold Blanc,Pierre Bobin, Denis Daumerie, Guy Discamps, JacquesGrosset, Gerard Grossetete, Jean Alain Husser, PierreJamet, Baohong Ji, Max Nebout, Steffan Pattyn, andIssa Traore.
Reprint requests to: Dr. Jacques Grosset, Bactériologle et Virologie, Faculté de Médecine Pitié-Salpê-trière, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
Received for publication on 5 May 1992.
Acceptedfor publication on 11 June 1992.