- Volume 60 , Number 4
- Page: 536–41
Does isoniazid increase the hepatotoxicity of the combination prothionamide-dapsone?
ABSTRACT
In order to assess the potential additive liver toxicity of isoniazid to that of a thioamide-containing treatment, a prospective, randomized, double-blind trial of 24 weeks' duration involving 772 adult patients was conducted in four leprosy centers-two in India, one in Madagascar, and one in the Ivory Coast. Patients with multibacillary leprosy were given daily 100 mg dapsone (DDS) and 350 mg prothionamide (PTH) plus monthly 600 mg rifampin (RMP) in combination either with 350 mg isoniazid (INH) or with a placebo. After clinical and laboratory (including HBs-Ag testing) examinations on admission, the side effects (especially gastrointestinal disturbances and liver toxicity) were assessed at regular intervals during treatment by laboratory testing (aminotransferases, bilirubin, alkaline phosphatase) and by recording spontaneous complaints. Analysis of the frequency and seriousness of the side effects was made before breaking the code (with or without INH). Although 10% of the patients had liver toxicity leading to stopping treatment, no significant difference in the occurrence of side effects was observed between patients treated with or without INH. Most (75%) of the observed side effects occurred during the first 4 weeks of treatment, and the time of their onset was not related to INH. Body weight and age were factors related to the frequency of side effects [the higher the body weight, the lesser the rate of side effects (p = 0.03)] and the rate of serious side effects increased with age (p = 0.02). But, again, the frequency of the side effects was not related to INH administration. Therefore, f rom the present study it can be concluded that INH does not increase the toxicity of the thioamide-containing treatment.RÉSUMÉ
Une étude prospective randomisée en double-aveugle d'une durée de 24 semaines comprenant 772 patients adultes a été réalisée dans quatre centres de lèpre deux en Inde, un à Madagascar et un en Côte d'Ivoire- pour évaluer la toxicité hépatique supplémentaire de l'isoniazide dans un traitement contenant un thioamide. Des patients présentant une lèpre multibacillaire ont reçu une dose quotidienne de 100 mg de dapsone (DDS) et 350 mg de prothioamide (PTH), ainsi qu'une dose mensuelle de 600 mg de rifampicine (RMP) combinée soit à 350 mg d'isoniazide, soit à un placebo. Après que des examens clinique et de laboratoires (y compris recheche de l'antigène HBS) aient été effectués à l'admission, les effets secondaires (particulièrement les troubles gastro-intestinaux et la toxicité hépatique) ont été évalués à intervalles réguliers durant le traitement par des tests de laboratoire (aminotransférascs, bilirubine, phosphatase alcaline) et par l'enregistrement des plaintes spontanées. L'analyse de la fréquence et de l'importance des effets secondaires a été réalisée avant que le code (avec ou sans INH) ne soit dévoilé. Bien que 10% des patients aient présenté une toxicité hépatique ayant amené l'arrêt du traitement, on n' a observé aucune différence significative dans la surveunue d'effets secondaires selon que les patients recevaient ou non de l'INH. La majorité (75%) des effets secondaires est survenue durant les quatre premières semaines de traitement, et le moment de leur apparition n'avait pas de rapport avec l'INH. Le poids corporel et l'âge étaient en rapport avec la fréquence des effets secondaires (plus grand le poids du corps, moins fréquents les effets secondaires [p = 0.03]), et la fréquence d'apparition d'effets secondaires sérieux augmentait avec l'âge (p = 0.02). Mais de nouveau, la fréquence des effets secondaires n'était pas associée à l'administration d'INH. On peut donc conclure de la présente étude que l'INH ne augmente pas à la toxicité d'un traitement contenant des thioamides.RESUMEN
Para establecer la toxicidad potencial sobre el hígado de la isoniazida adicionada a un tratamiento conteniendo una tioamida, se hizo un estudio aleatorio, prospectivo, a doble ciego, de 24 semanas de duración, en 772 pacientes adultos de 4 centros antileprosos (2 en la India, uno en Madagascar, y uno en Costa de Marfil). A los pacientes con lepra multibacilar se les DIO diariamente 100 mg de dapsona (DDS) y 350 mg de protionamida (PTH) más 600 mg, una vez al mes, de rifampina (RMP) en combinación con 350 mg de isoniazida (INH) o con un placebo. Una vez iniciado el tratamiento, a intervalos regulares se estudiaron sus efectos colaterales, especialmente los disturbios gastrointestinales y la toxicidad hepática. Para esto se utilizaron el criterio clínico, los datos de laboratorio (aminotransferasas, bilirrubina y fosfatasa alcalina), y los registros de las quejas espontaneas de los pacientes. El análisis de la frecuencia y severidad de los efectos colaterales se hizo antes de descodilícar los datos. Aunque el 10% de los pacientes tuvieron toxicidad hepática (lo cual condujo a suspender el tratamiento), no se observaron diferencias significativas en la ocurrencia de los efectos colaterales en los pacientes tratados con o sin INH. La mayoría (75%) de los efectos colaterales observados ocurrieron durante las primeras 4 semanas de tratamiento pero el tiempo de su aparición no estuvo relacionado con la INH. El peso corporal y la edad fueron factores relacionados con la frecuencia de los efectos colaterales: mientras mayor fue el peso corporal, menor fue la frecuencia de efectos colaterales (p = 0.03) y la frecuencia de éstos aumentó en forma proporcional a la edad (p = 0.02). Pero otra vez, la frecuencia de los efectos colaterales no estuvo asociada con la administración de INH. Por lo tanto, del presente estudio se puede concluir que la INH no aumento a la toxicidad del tratamiento conteniendo una tioamida.In many countries a combined formulation containing (in a single tablet) 50 nig dapsonc (DDS), 175 mg prothionamide (PTH) and 175 mg isoniazid (INH) is used for the treatment of both leprosy and tuberculosis. This formulation is useful under field conditions when combined with rifampin (RMP). The three drugs DDS, PTH, and RMP are active against Mycobacterium leprae and the three drugs INH, PTH and RMP are active against M. tuberculosis. Despite its advantage for the management of field treatment (three drugs in the same tablet), the combination of DDS, PTH and INH is not universally accepted because it always contains one drug-INH for leprosy and DDS for tuberculosis-the activity of which against the responsible organism is doubtful. INH has some potential liver toxicity (6), and DDS has the tendency to create methemoglobinemia (4, 9). Therefore, it is important to measure comparatively the side effects in leprosy patients treated with and without INH to determine whether the patients receiving the combined formulation are not running an undue risk of toxicity.
In the literature minor side effects have been observed in up to 45% of patients receiving a thioamide (12). The side effects observed with ethionamide as well as prothionamide (1) are mainly gastrointestinal: metallic taste, nausea, gastric discomfort, anorexia and vomiting. The side effects are regarded as responsible for the bad compliance of patients prescribed a thioamide (5, l3). A major side effect, liver toxicity that occurs mainly in the first 6 months of treatment, is reported in an average proportion of 2%-4% (2, 10) when RMP is not given simultaneously, but in a proportion of up to 56% when given daily in combination with RMP or another rifamycin derivative (2, 8, 11).
To assess the potential additive liver toxicity of INH, a multicenter study was planned in which 1000 patients with active and inactive multibacillary (MB) leprosy were to be randomized in two groups and treated for 24 weeks either with daily Isoprodian® plus monthly RMP or with daily DDS and PTH plus monthly RMP. The study was to be conducted under doubleblind conditions, and the intake of the drugs was to be fully supervised. The incidence of minor (not leading to stopping treatment) and major (leading to stopping treatment) side effects was assessed by a self-report form from the patient and by weekly questioning for minor side effects and by liver function tests and clinical examinations done every 2 weeks.
MATERIALS AND METHODS
Selection of patients. The patients included in the study were all over 15 years of age but not older than 65, and were prepared to fulfill the conditions of the study, to remain in the same area and to accept daily fully supervised treatment. All of them had not received multidrug therapy previously. They had MB leprosy, either active [bacterial index (BI) of 2+ or more in at least one site] or inactive but known from the patient's record file (with at least a BI of 2+ or more twice in the history).
The following patients were not included: pregnant females, patients with HBs-Ag positive, patients with aminotransferase levels three or more times the upper limit of normal, patients suffering from an organic or psychiatric disease that might interfere with the present study, patients whose body weight was below 40 kg, patients with known allergic or toxic reactions against one of the components of Isoprodian® or RMP, patients with macrohematuria and patients with smear-positive tuberculosis.
Methods. On admission each patient had a complete clinical history and physical examination. Blood samples were obtained for laboratory studies including hemoglobin, aminotransferases, bilirubin, alkaline phosphatase, and HBs-Ag. Urine specimens were obtained for a red blood cell count and protein detection. When possible, a posterioranterior chest X-ray was made, and in case of the presence of abnormal X-ray shadows, sputum smears were done to confirm the diagnosis of tuberculosis.
The patients were treated with either the INH-containing regimen or the regimen not containing INH, randomization being made at the time of preparation of the tablet bottles. Whatever the body weight, each took every day two Isoprodian® tablets or two similar tablets each containing 50 mg DDS and 175 mg PTH without INH and every month two 300 mg tablets of RMP, and swallowed all of them under supervision during the 24 weeks of the study.
During treatment, every day at the time of administration, no questions concerning acceptability of the drugs were asked. However, spontaneous complaints were recorded; every week, standard questioning not intended to induce complaints was performed. Every 4 weeks, aminotransferase levels were measured. As long as the values remained within acceptable range (below three times the upper normal limit), the aminotransferase tests continued to be done every 4 weeks. If the values were three to five times higher than normal, the aminotransferase tests were repeated within the next 2 weeks without change in chemotherapy. If the values were more than five times higher than normal, all drugs were discontinued, and the diagnosis of hepatitis was made. When laboratory tests returned to normal, the treatment was resumed with the WHO-recommended regimen, dapsone, clofazimine and rifampin. In the case of immune reactions, adequate treatment was undertaken without changing the specific treatment.
An analysis of the frequency and seriousness of the side effects was made before breaking the code (with or without INH). Statistical analysis was conducted according to the following tests: a) chi-squared test to describe a connection between two variables; if necessary, this was replaced by the Fisher-Yates test; b) multiple regression analysis to describe the influence of all variables on the incidence of side effects; c) Kaplan-Meyer and log-survival statistics to describe the time of onset of side effects. Results with p values < 0.05 were considered significant.
RESULTS
Because of administrative reasons, two centers (one in Africa, one in South America) did not participate in the study. Only 772 patients from 4 centers (2 in India, 1 in Madagascar, and 1 in the Ivory Coast) instead of the 1000 expected patients have been included in the study. Among them, 78 were excluded for reasons other than side effects, and 694 remained eligible for assessment.
Global results. During the 24 weeks of treatment, 367 patients (52.8%) suffered mild side effects which did not lead to stopping treatment and 113(16.2%) suffered side effects considered serious enough either by the patient or by the doctor in charge to stop treatment. As shown in Table 1, the rate of mild side effects ranged from 45.9% in Adzope, Ivory Coast, to 58.7% in Manankavaly, Madagascar. The rate of serious side effects which led to stopping treatment ranged from 6.9% in Adzope to 25.3% in Manankavaly. In the latter two centers, the serious side effects were limited to liver toxicity, and the decision to stop treatment was made only by a doctor. In Madras and Poona, stopping treatment was due to liver toxicity and to other causes, mainly gastrointestinal disturbances, and was a decision of the patients as well as the doctors. In Madras, stopping treatment was a decision made 18 times by patients and 21 times by doctors, and in Poona 10 times by patients and 34 times by doctors. Because of the high rate of hepatitis, the study was prematurely terminated in Manankavaly, Madagascar, before the planned number of patients to be included was reached. All patients with hepatitis recovered when the drugs were stopped.
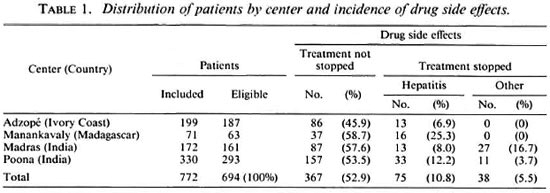
Analytical results. After breaking the code, there was no significant difference in the occurrence or nonoccurrence of mild as well as serious side effects when patients treated with INH were compared to those not treated with INH (Table 2). Such a conclusion holds true for patients in an individual center as well as in the four centers considered together.
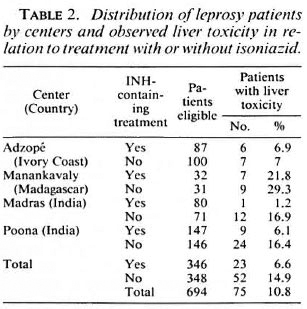
Most (75.3%) of the side effects occurred within the first 4 weeks of treatment. Then, 10.1% occurred between 4 and 8 weeks, another 10% between 8 and 16 weeks, and 4.4% between 16 and 24 weeks. There was no difference in the onset of side effects in relation to whether or not the patients were treated with INH.
Patients (N = 75) with a previous history of jaundice did not have significantly more mild or serious side effects than those without a previous history of jaundice, whether or not treated with INH. There is also no suggestion that the 247 patients with a previous history of erythema nodosum leprosum (ENL) or the 55 patients with ENL during treatment were more at risk of side effects.
Serious side effects occurred in 80 (15.1%) of the 530 males and in 33 (20.1%) of the 164 females. Although the results suggested that females might be more at risk of side effects, the difference was not statistically significant, whether or not they were treated with INH.
As shown in Table 3, the incidence of side effects was related to the body weight of patients. The higher the body weight, the lesser the rate of serious side effects. The difference is statistically significant (p = 0.03) between patients with a body weight more than 60 kg and patients with a body weight of 41 kg to 60 kg. There was no difference whether the patients were or were not treated with INH.
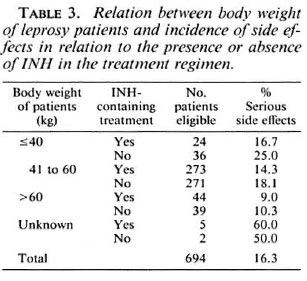
The rate of serious side effects increased with age. It was 7.8% among the 103 patients aged less than 25 years, 17.2% among the 513 aged between 25 and 54 years, and 21.8% among the 78 aged 55 years or more. The difference between the patients of the youngest age group and the others is statistically significant (p = 0.02). Again, INH did not contribute to increase the rate of side effects.
Finally, multiple regression analysis did not show a direct relationship between the intake of INH and the occurrence of side effects. Body weight and age are the only factors which seem to have had an influence on the occurrence of side effects.
DISCUSSION
The objective of the present study was to measure the contribution of INH to the overall tolerance and toxicity of a combined formulation containing PTH, DDS and INH in the treatment of lepromatous leprosy. By randomization, half of the patients were given the three drugs combined in single tablets of Isoprodian® and the other half were given PTH and DDS in similar tablets. Neither patients nor doctors were aware of the composition of the tablets, and the daily drug intake was fully supervised. Evaluation of the side effects was made before the randomization code was broken. Since no statistically significant differences were noted in the rate of side effects between those patients treated with INH and those treated without INH, it can be concluded that INH did not contribute, in the present study, to the overall intolerance and toxicity of the combined formulation as already suggested by Ellard, et al. (5). Although INH is a drug well known for its potential hepatotoxicity (6), its individual effects may have been diluted in the much more potent toxicity of prothionamide of which the reported risk of liver toxicity is of the same order of magnitude as those presently observed (3, 8, 11)
However, the present study suffered two limitations. First, out of the 6 centers that accepted to participate (3 in Africa, 2 in India, 1 in South America), only 4 actually participated (1 in main Africa, 1 in Madagascar, and 2 in India). As a result, only 694 patients were eligible for analysis, instead of the expected 1000, and this might not represent the complete range of tolerance and toxicity to the combination PTH, DDS and INH. In addition, since the rate of major side effects ranged from 6.9% in the Ivory Coast to 25.3% in Madagascar, extrapolation to other places of the world should be made with care. Geographical differences in the rate of side effects have also been reported with thiacetazone, again, a drug with a sulfur atom (8).
Second, neither the daily nor the monthly drug dosages were adapted to the body weight of the patients. This is inherent in the fixed combination tablets that were used, and of which the main advantage is to prevent irregular intake of individual drugs, but this may have contributed to the overall proportion of the side effects. Since the majority of the patients had a body weight between 41 kg and 60 kg, the daily doses of 350 nig PTH and 100 mg DDS might have been somehow too high (13). The fact that the incidence of serious side effects increased when the mean body weight of patients decreased is an argument in favor of a drug dosage that was too high for the majority of the patients. It is, therefore, possible that in countries where the bociy weight of patients is higher than that of the patients included in the present study, the rate of side effects would be lower. Another possibility is that, in routine practice, patients treated with PTH are suffering gastrointestinal side effects, and thus are less compliant in their intake of drugs than those who are not treated with PTH and, consequently, escape major liver toxicity, as suggested by reports from India(5, l3). These possibilities may explain why reports on the tolerance and toxicity of the combination of DDS, PTH, and INH used in field conditions are so different.
A point of interest is the time of onset of the side effects. More than 75% of the side effects occurred during the first 4 weeks of treatment. This confirms that toxicity to PTH is not cumulative and might well result from an individual's susceptibility to the drug or its sulfur atom (3). In the case of a thioamide-containing treatment, a way to detect toxicity and to prevent serious liver damage (11, 14) would be to keep the patient under strict surveillance and to perform weekly liver function tests during the first month of treatment.
Acknowledgment. This investigation received financial support from the DHA W in Wiir/burg, Germany. Tablets without isoniazid were kindly supplied by Messrs. Fatol Arzneimittel. Schitfweiler. The authors are grateful to Dr. (Ms) Like Egert for expert assistance in the statistical analysis.
REFERENCES
1. BRITISH TUBERCULOSIS ASSOCIATION RESEARCH COMMITTEE. A comparison of the toxicity of prothionamide and ethionamide. Tubercle 49(1968)125-135.
2. CARTEL, J.-L., MILLAN. J.. GUELPA-LAURAS, C.-C. and GROSSET, J.-H. Hepatitis in leprosy patients treated by a daily combination of dapsone, rifampin, and a thioamide. Int. J. Lepr. 51(1983)461-465.
3. CARTEL, J.-L., NAUDILLON, Y., ARTUS, J. C. and GROSSET, J.-H. Hepatotoxicity of the daily combination 5 mg/kg prothionamide + 10 mg/kg rifampin. Int. J. Lepr. 53(1985)15-18.
4. DAVIES, R. Fatal poisoning with Udolac (diaminodiphenylsulphone). Lancet 1(1950)905-906.
5. ELLARD, G. A., KIRAN. K. U. and STANLEY, J. N. A. Long-term compliance: a study carried out in India using a combined formulation containing prothionamide. dapsone and isoniazid. Lepr. Rev. 59(1988)163-175.
6. GIRLING, D.J. Adverse effects of antituberculosis drugs. Drugs 23(1982)56-74.
7. GROSSET, J. and BENHASSINE, M. La thiacétazonc: données expérimentales et cliniques récentes. Adv. Tuberc. Res. 17(1970)107-153.
8. Ji, B.. CHEN. J.. WANG , C. and XIA, G. Hepatotoxicity of combined therapy with rifampicin and daily prothionamide for leprosy. Lepr. Rev. 55(1984)283-289.
9. MANDRELL, G. L. and SANDE, M. A. Antimicrobial agents (continued); drugs used in the chemotherapy of tuberculosis and leprosy. In: Goodman and Oilman's The Pharmacological Basis of Therapeutics. 6th edn. New York: Macmillan Publishing Co., 1980, pp. 1214-1215.
10. MARTIN-LALANDE, J., JAUBERTIE, R., DJEBBAR, A. and PHAM, Q. T. Etude clinique et biologique de la tolérance au tuberculostratiquc 1321 Th (prothionamide). Rev. Tuberc. Pneum. (Paris) 30(1966)1233-1244.
11. PATTYN, S. R., JANSSENS, L., BOURLAND, J., SAYLAN, T., DAVIES, E. M., GRILLONE, S., FERACCI, C. and THE COLLABORATIVE STUDY GROUP FOR THE TREATMENT OF LEPROSY. Hepatoxicity of the combination of rifampin-ethionamide in the treatment of multibacillary leprosy. Int. J. Lepr. 52(1984)1-6.
12. RIST, N. L'activité antituberculeuse de ('ethionamide. Adv. Tuberc. Res. 10(1969)69-126.
13. STANLEY, J. N. A., PEARSON, J. M. H. and ELLARD, G. A. Ethionamide, prothionamide and thiacétazonc self-administration; studies of patient compliance using isoniazid-marked formulations. Lepr. Rev. 57(1986)9-18.
14. WHO SPECIAL PROGRAMME FOR RESEARCH and TRAINING IN TROPICAL DISEASES. Fifth Annual Report. Leprosy. Chapter 8. Geneva: World Health Organization, 1981.
Members of the Study Group are, in alphabetical order: Horst Franck, M.D., German Leprosy Relief Association, Wurzburg, Germany. Jacques H. Grosset, M.D., Faculté de Médecine Pitié-Salpêtrièrc, 91 lilvd. de l'Hôpital. 75634 Paris 13. France. Christian Hupin, M.D., Institut Raoul Follereau, Antananarivo, Madagascar. Dick L. Leiker, M.D., Nederlandse Stichting Voor Leprabestrijding. Amsterdam, The Netherlands. Derek Lobo, M.D., Grcmaltes, Referral Hospital and Leprosy Center, Madras, India. Jal Mehta, M.D.. Poona District Leprosy Committee, Punc, India. Laurent N. N'Deli, M.D., Institut Raoul Follereau, Adzopé, Ivory Coast. Richard Urbanczik, M.D., Fatol Arzneimittel GmBH, Schiffweiler, Germany.
Reprint requests to Prof. J. H. Grosset.
Received for publication on 10 June 1992.
Accepted for publication in revised form on 31 August 1992.