- Volume 60 , Number 4
- Page: 542–8
Short-term trial of clofazimine in previously untreated lepromatous leprosy
ABSTRACT
Forty-five previously untreated lepromatous leprosy patients were allocated randomly to three groups and treated, respectively, with Regimen A, standard dosage of clofazimine (CLO) in multidrug therapy (MDT) regimen; Regimen B, CLO 600 mg once every 4 weeks; and Regimen C, CLO 1200 mg once every 4 weeks. The duration of the trial was 24 weeks. By the end of the trial, although a few patients in each group did not improve at all clinically, the majority of patients showed clinical amelioration but the responses were slow. While the mean morphological index dropped to the baseline after 24 weeks of treatment, the mean bacterial index did not change significantly. About 80% of the patients in each group remained nasal-smear positive at the end of the trial, but the bacterial loads steadily declined. No significant difference has been detected in these parameters among the three groups. The patients tolerated the regimens very well and the side effects were mild. The results of serial mouse foot pad inoculation demonstrated that the positivity rates of multiplication of Mycobacterium leprae in mice and the proportions of viable organisms reduced gradually in all groups. Because the positivity rate at week 24 in Group C did not differ significantly f rom Group A, but was significantly smaller than that of Group B, we conclude that Regimen C was as active as Regimen A and could be applied for monthly supervised treatment along with rifampin; Regimen B is less effective and should not be used for the treatment of leprosy.RÉSUMÉ
Quarante-cinq malades lépromateux non encore traités ont été répartis de manière aléatoire en trois groupes et traités respectivement avec: un régime A, consistant en de la clofazimine (CLO) à la dose standard utilisée dans les régimes de polychimiothérapie (PCT); un régime B, consistant en de la clofazimine 600 mg une fois toutes les quatre semaines; et un régime C, de la clofazimine à la dose de 1200 mg une fois toutes les quatre semaines. La durée de l'essai était de 24 semaines. A la lin de l'essai, bien que quelques patients dans chacun des groupes n'avaient bénéficié d'aucune amélioration clinique, la majorité des patients avait montré une amélioration, mais les réponses avaient été lentes. Alors que l'indice morphologique moyen tombait à zéro après 24 semaines, l'indice bactérien moyen n'a pas changé de manière significative. Environ 80% des patients dans chaque groupe ont gardé des frottis positifs au niveau des fosses nasales à la fin de l'essai, mais les charges bactériennes diminuaient régulièrement. On n'a pas détecté de différence significative entre les trois groupes du point de vue de ces paramètres. Les patients ont très bien toléré les régimes, et les effets secondaires ont été légers. Les résultats d'inoculation en série dans le coussinet plantaire de souris ont montré que le taux de positivite de la multiplication de Mycobacterium leprae chez la souris et la proportion d'organismes viables ont diminué progressivement dans tous les groupes. Comm e le taux de positi vite à 24 semaines pour le groupe C ne différait pas significativement de celui du groupe A, mais était significativement plus petit que celui du groupe B. nous en concluons que le régime C est aussi actif que le régime A et peut être appliqué pour un traitement mensuel supervisé associé à la rifampicine; le régime B est moins efficace et ne ne devrait pas être utilisé pour le traitement de la lèpre.RESUMEN
Cuarenta y cinco pacientes con lepra lepromatosa nunca antes tratada, se agruparon al azar en tres grupos y se trataron respectivamente, con los siguientes esquemas de tratamiento: (A) la dosis estándar de clofazimina (CLO) usada en la poliquimioterapia (PQT), (B) 600 mg de CLO cada 4 semanas, y (C) 1,200 mg de CLO cada 4 semanas. La duración del tratamiento fue de 24 semanas. Al finalizar el ensayo se encontró que no obstante que algunos pacientes de cada grupo no mostraron ninguna mejoría clínica, la mayoría de los pacientes sí mejoraron clínicamente, aunque las resupuestas fueron lentas. Mientras que el índice morfológico promedio decayó hasta el nivel basal después de las 24 semanas de tratamiento, el índice bacteriano no cambió significativamente. Aproximadamente el 80% de los pacientes de cada grupo mostraron bacilos en los raspados nasales, pero el número de bacilos estuvo en constante decaimiento. No se encontraron diferencias en estos parámetros entre los diferentes grupos, los pacientes toleraron muy bien los diferentes esquemas de tratamiento y los efectos colaterales fueron moderados. Los resultados de las inoculaciones seriadas en las almohadillas plantares del ratón, demostraron que la capacidad de multiplicación del Mycobacterium leprae, y la proporción de bacilos viables, declinaron gradualmente en todos los grupos. Puesto que al final del tratamiento (semana 24) el grado de positividad en el grupo C no difirió significativamente de la positividad en el grupo A, pero fue mucho menor que aquella del grupo B, se concluye que el esquema C es tan efectivo como el esquema A y que puede ser aplicado, junto con rifampina, como un tratamiento con supervisión mensual. El tratamiento B es menos efectivo y no debe usarse para el tratamiento de la lepra.Because of its bactericidal activity against Mycobacterium leprae (2, 16)and promising effect in the treatment of erythema nodosum leprosum (ENL) (1, 4) , clofazimine (CLO) is one of the important components of multidrug therapy (MDT) for multibacillary (MB) leprosy as recommended by the WHO Study Group (17). It was recommended to treat the MB patients with CLO 50 mg daily, unsupervised, and 300 mg once monthly, supervised, together with monthly rifampin (RMP) 600 mg and daily dapsone (DDS) 100 mg (17). The main objective of administration of both CLO and DDS in this regimen is to prevent the selection of RMP-resistant mutants (8).
However, it is well known that, as in most chronic diseases, many leprosy patients do not take their prescribed drugs regularly unless the administration of drugs is supervised. A recent study demonstrated that close to 30% of the patients in Karigiri, one of the best leprosy control programs in the world and where CLO is well accepted by the patients because of their dark skin, did not take their prescribed DDS and CLO properly (3), suggesting that simply because of noncompliance it is still possible to develop RMP resistance in a program where MDT has been implemented. The potential risk is likely to be more serious among lightskinned patients because many of them do not readily accept CLO due to the skin coloration it causes. The risk from noncompliance may be significantly reduced if a fully supervised MDT regimen or at least two of the three components of the current regimen are administered under supervision once monthly.
In view of the extraordinarily long halflife (t½) of CLO in humans, approximately 70 days (10), it is very likely that the drug may be administered once monthly under supervision as RMP, and thereby prevent the emergence of RMP resistance due to noncompliance of the patients. To date, studies on the efficacy of intermittent CLO treatment are limited (17); no results on monthly CLO treatment have ever been reported. Several hundreds of patients in Shanghai, China, have been treated with a modified MDT regimen in which CLO was administered at 1200 mg once monthly (Ji, B., personal communication), but it is unclear whether the bactericidal activity of monthly CLO is as active as the dosage of CLO in the stardard MDT regimen.
The objectives of this study were to compare: a) the clinical response and bactericidal activities against M. leprae between standard CLO regimen and the two monthly administered CLO (600 mg or 1200 mg) regimens; and b) the severity of the side effects between the three regimens.
PATIENTS AND METHODS
Patients. Between January 1987 and May 1988, 45 newly diagnosed lepromatous (LL and BL) patients with active leprosy lesions and high bacterial loads were recruited into the trial. During their first visit to our clinic, all of them denied previous antileprosy treatment, and their urine dapsone analyses were negative. Among the 45 cases, 31 were male, 14 female; age ranged from 15 to 64, with mean ± standard deviation of 30.2 ± 11.4, years old at intake. Based on clinical classification, 39 were LL and 6 BL leprosy. After admission, they were allocated randomly to three groups: Group A, 16 cases; Group B, 13 cases; and Group C, 16 cases. All of the patients were hospitalized during the trial.
Examinations at intake and during the trial. The examinations employed in the trial basically followed the THELEP standard protocol for clinical trials in lepromatous leprosy (14, 15), except that the bactericidal activity of the treatment in our trial was monitored by serial, 10-fold-diluted inocula into mouse foot pads (5).
In brief, the following examinations were carried out on each patient before treatment (WO): a thorough physical examination, especially the examination for leprosy; lepromin test; posterior-anterior chest X-ray; skin smears from six sites of lesions and a nasal (nose blow) smear for measurement of the bacterial index (BI) and morphological index (MI); blood and urine analysis; liver function (SGPT, serum bilirubin and alkaline phosphatase) and renal function (blood urea nitrogen and creatinine) tests; and a skin biopsy for mouse foot pad inoculation from the most active lesion which was large enough to provide several biopsies during the trial.
Every 4 weeks during the trial, clinical examinations, blood and urine analysis, and liver and renal function tests were repeated, and the patients were interviewed for symptoms suggesting adverse reactions to CLO. Based on changes of clinical manifestations as compared with WO, clinical response was assessed every 4 weeks during treatment and was scored as 0 (no change), 1 + (definite improvement), and 2+ (marked improvement) (5). Skin smears were repeated from the same sites at the end of the trial, whereas the nasal (nose blow) smear was repeated every 4 weeks during treatment. Skin biopsy, taken from the same lesion as WO, was repeated every 8 weeks for mouse foot pad inoculation.
Mouse foot pad inoculation. There were two different purposes for the pretreatment biopsies: a) to demonstrate viable organisms with different inocula before treatment; and b) to determine the minimal effective dosage (MED) of CLO against M. leprae. For those biopsies taken during treatment, the only purpose was to demonstrate viable organisms with different inocula.
The procedures for demonstrating viable organisms by mouse inoculation have been described at length (5). In the current trial, the organisms recovered from pretreatment (WO) specimens and biopsies taken at 8 weeks (W8) during treatment were inoculated into immunocompetent mice at three different concentrations, 5 × 103 5 × 102 and 5x101 acid-fast bacilli (AFB) per foot pad, and two concentrations, 5 × 103 and 5 × 102 AFB per foot pad, were inoculated from biopsies taken at 16 and 24 weeks (W16 and W24). Ten mice were inoculated per dilution, and the foot pads were harvested at 12 months after inoculation. The criterion of multiplication of M. leprae was that > 105 organisms per foot pad had been harvested. Any of the harvested foot pads with the same inoculum showing multiplication of M. leprae was considered as a positive mouse inoculation for the given inoculum. For the measurement of MED, immunocompetent mice were inoculated with 5 × 103 organisms per foot pad and allocated into four groups of 10 mice each: untreated control, and treated, respectively, with diets containing 0.0001%, 0.001% and 0.01% of CLO by the continuous method (12). The MED was defined as the lowest concentration of CLO administered in mice in which multiplication of M. leprae (> 105) was not observed in a single foot pad, while multiplication was observed in a significant proportion of foot pads in the control group (9).
Treatment. Patients from Groups A, B, and C were treated, respectively, with Regimens A, B, and C: Regimen A (standard CLO regimen) = CLO 50 mg daily plus 300 mg once every 4 weeks; Regimen B = CLO 600 mg once every 4 weeks; and Regimen C = CLO 1200 mg once every 4 weeks. All the drugs were administered under supervision. Unauthorized RMP is not accessible to the patients in our institute.
Statistical analysis. The data were analyzed by means of the paired sample Student's / test for comparison of mean values, and by Fisher's exact probability test for comparison of frequencies.
RESULTS
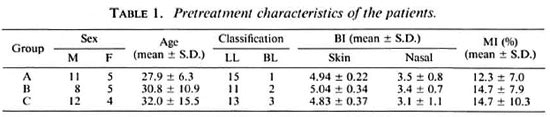
Pretreatment characteristics of the patients. The major pretreatment characteristics of the patients in the groups are presented in Table 1. They did not differ significantly (p > 0.05) and, therefore, the three groups were comparable.
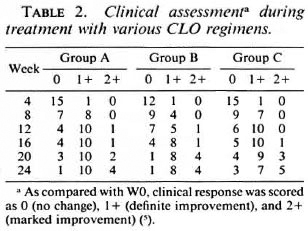
Clinical responses during treatment. The clinical assessments of the patients during treatment are summarized in Table 2. A few patients in each group did not improve at all clinically, but the majority of patients showed clinical improvement at W24. The responses were slow: after 12 weeks oftreatment, 4 of 15 cases in Group A, 7 of 13 in Group 13, and 6 of 16 in Group C still did not show any evidence of clinical improvement; at W24, marked improvement (2+) was found in no more than one third of the patients in each group. At any time, the clinical responses in Groups B and C did not differ significantly from that of Group A (p > 0.05).
Changes of BI and MI of skin and nasal smears. While the mean MI of each group fell significantly to the baseline (p < 0.01) after 24 weeks of treatment, and the reduction did not differ significantly among the three regimens, a slight but significant reduction of the mean BI was observed only in Group B (p < 0.05). With respect to the nasal smears, 11 of 14 cases in Group A, 10 of 13 in Group B, and 12 of 15 in Group C (about 80% of patients in each group) remained positive after 24 weeks of treatment. However, in terms of BI, the mean bacterial loads in nasal smears progressively declined in all groups, the results at W24 were at least 1 + smaller than those at W0, and the differences were highly significant (p < 0.01). The results are summarized in Table 3.

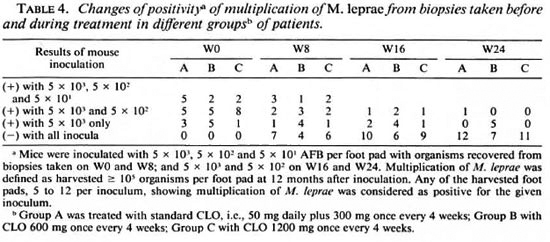
Changes of positivity rates of multiplication of M. leprae in the mouse. Among 43 patients who completed the trial, the organisms of pretreatment biopsies from 7 patients-2 of 15 in Group A, 1 of 13 in Group B, and 4 of 15 in Group C-failed to multiply in the foot pads of mice. The overall failure rate, 16.3%, is similar to those of earlier trials conducted in our institute and in Chingleput by THELEP (13). These patients were excluded from the analysis of results of serial mouse foot pad inoculation.
In five patients (nos. 7, 13, 17, 19 and 24) the serial inoculation displayed alternative negative and positive results during treatment. In order to avoid an overestimate of the activity of the treatment, and because false-positive growth in the mouse foot pad is far less common than a false-negative result with the criterion of multiplication employed in our trial, we therefore consider the earlier negative results as positives. The positivity of multiplication of M. leprae of biopsies taken at different intervals from the groups is summarized in Table 4. The proportions of positives gradually declined, indicating that a certain proportion of M. leprae were killed during the course of treatment. The positivity rate at W8 was significantly less than that of W0 in Group A (p < 0.01), B (p < 0.05), and C (p < 0.01). Nonetheless, similar to the clinical responses described earlier, the speed of negativity of mouse inoculation was relatively slow. At W8, close to half of the biopsies from Groups A and C and two thirds of the specimens from Group B were still positive. At W24, although the organisms from the great majority ofpatients in Groups A and C had lost their infectivity to mice, the organisms from 40% of the patients in Group B were still able to multiply in mice. At this point, the positivity rate of Group C was significantly less than that of Group B (p < 0.05), and the difference of positivity rates between Group A and B was just beyond the limit of significance (p = 0.058).
Estimation of bactericidal activities against M. leprae by the treatment. Based on the results of multiplication in Table 4, the median result was positive after inoculation with 5 × 102 AFB per foot pad in all three groups before treatment. Thus, the median value of the proportion of viables was about 1 in 500. After 24 weeks of treatment, the median result was negative in all three groups after inoculation with 5 × 103 AFB per foot pad. Thus, the median value of proportion of viables decreased to less than 1 in 5000. These results suggested that after 24 weeks of treatment, all three regimens killed about one log10 of viables.
Because only three different dilutions (5 × 103, 5 × 102, and 5 × 101 AFB per foot pad) of inocula were used, using the Spearman-Karber method for calculating the median infectious dose (ID50) (11), the proportion of viables can only be estimated up to the limit of > 4.35% (all positives in three dilutions) or down to < 0.006% (all negatives in three dilutions) (5). Based on individual results of inoculation (data not shown), the mean proportion of viables in the pretreatment biopsies was 0.46 ± 1.2% in Group A, 0.12 ± 0.26% in Group B, and 0.12 ± 0.15% in Group C. After 24 weeks of treatment, the proportion of viables ranged from < 0.006% to 0.01% in Group A, from < 0.006% to 0.022% in Group B, and < 0.006% in Group C. The median values (and their ranges) of the proportion of viables killed by the treatment were > 88.9% (> 50.0% to > 99.9%) in Group A; 63.6% (0% to > 99.3%) in Group B, and > 90% (> 25% to > 98.6%) in Group C.
Determination of MED of CLO against M.leprae. None of the 36 strains which showed multiplication in the control group multiplied in mice fed with a CLO-containing diet, suggesting that 0.0001% in the mouse diet is likely to be the MED of CLO.
Leprosy reaction. Reversal reactions were observed in 10 cases during the trial: 2 in Group A, 1 in Group B, and 7 in Group C. Therefore, it seems CLO in the doses utilized in this trial cannot prevent the development of a reversal reaction. The frequency of reversal reaction in Group C was significantly greater than the frequencies in the other two groups (p < 0.05). It is unclear why close to 50% of the patients in Group C developed reversal reactions. Most of the reactions were moderate in severity and responded well to corticosteriods. Only two patients, one each in Groups B and C, developed mild ENL during the trial.
Side effects of the treatments. Because of the dark skin of all our patients, skin coloration caused by CLO was not evident and never became a problem for the acceptability of the treatment. No gastrointestinal side effects were observed in any of these patients during treatment. Ichthyosis was seen in 11 cases: 5 in Group A, 3 each in Groups B and C; its frequency did not differ significantly among the groups. Despite the monthly repeating of various laboratory examinations, no abnormality was observed in any of the patients during the trial.
DISCUSSION
To our knowledge, the activity of the CLO component in the standard MDT regimen has never been evaluated in detail. The results found in Group A patients in the current trial clearly demonstrate that the standard dosage of CLO is active, resulting in clinical amelioration, reduction of the MI, killing of M. leprae, and also is very safe. However, clinical amelioration was slow and the bactericidal activity against M. leprae was moderate, about 90% (one log10) of viable organisms were killed after 24 weeks of treatment. Therefore, it is justified to conclude that CLO is weakly bactericidal against M.leprae, especially when compared with fluoroquinolones (5), clarithromycin and minocycline (6), which killed 3 or 4 log10 of viable organisms during the first 4 weeks of treatment.
Among the two monthly dosages of CLO tested, it is encouraging that by any parameters employed in the current trial no difference in efficacy could be detected between the standard daily CLO (Regimen A) and CLO 1200 mg once every 4 weeks (Regimen C). Furthermore, the tolerance of the monthly administration of CLO 1200 mg was as good as that of the standard CLO regimen. In order to improve the compliance of MDT, it is possible to give CLO 1200 mg once monthly together with monthly RMP. Because at W24 the positivity rate of multiplication of M. leprae in mouse foot pads with organisms recovered from Group B (treated with CLO 600 mg once every 4 weeks) was significantly greater than Group C (p < 0.05), and the difference from Group A was just beyond the limit of significance, CLO 600 mg once every 4 weeks seems to be less active than the other two regimens and should not be applied in the field for the treatment of leprosy.
The positivity rate of multiplication of M. leprae in mouse foot pads at W24 was 1/13 for Group A and 0/11 for Group C. When compared with the only published results on efficacies of intermittent CLO treatments (16), the figures of our two groups were similar to those treated with CLO 200 mg 6 times weekly (0/9), CLO 100 mg three times weekly (0/10), CLO 300 mg once weekly (1 / 10), and CLO 600 mg once every 2 weeks (2/6) (p > 0.05). The positivity rate of our Regimen C was significantly less than that of CLO 600 mg on two consecutive days every 4 weeks (4/10) (p < 0.05), but our Regimen A did not differ significantly from the latter regimen (p > 0.05).
Although CLO is an established antileprosy drug, its minimal effective dose (MED) against M. leprae in mice previously had not been well documented (7). As a by-product of the trial, we have demonstrated that all 36 strains of M. leprae did not multiply in mice fed with diet containing 0.0001% CLO, suggesting that 0.0001 % in mouse diet is likely to be the MED of CLO, and may be employed as the criterion for the diagnosis of CLO-resistant M. leprae.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases.
REFERENCES
1. BROWNE, S. G. "B663" -a possible anti-inflammatory action in lepromatous leprosy. Lepr. Rev. 36(1965)9-11.
2. BROWNE, S. G. and HOGERZEIL, L. M. "B663" in the treatment of leprosy; preliminary report of a pilot trial. Lepr. Rev. 33(1962)6-10.
3. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN, M. Clofazimine and dapsone compliance in leprosy. Lepr. Rev. 59(1988)205-223.
4. HASTINGS, R. C. and TRAUTMAN, J. R. B663 in lepromatous leprosy; effect in erythema nodosum leprosum. Lepr. Rev. 39(1968)3-7.
5. GROSSET, J.-H., JI, B., GUELPA-LAURAS, C.-C , PERANI, E. G. and N'DELI, L. N . Clinical trial of petloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281 -295.
6. JAMET, P., JI, B., BOBIN, P. and GROSSET, J.-H. Powerful bactericidal activities of clarithromycin and/or minocycline against M. leprae in man. (Abstract no. 970) 31st ICAAC, September 29-October 2, 1991, Chicago, Illinois.
7. Ji, B. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
8. Ji, B. and GROSSET, J.-H. Recent advances in the chemotherapy of leprosy. (Editorial) Lepr. Rev. 61(1990)313-329.
9. Ji, B., MATSUO, Y. and COLSTON, M . J. Screening of drugs for activity against Mycobacterium leprae. Int. J. Lepr. 56(1987)836-842.
10. LEVY, L. Pharmacologic studies of clofazimine. Am. J. Trop. Med. Hyg. 23(1974)1097-1109.
11. SHEPARD, C. C. Statistical analysis of results obtained by two methods for testing drug activity against Mycobacterium leprae. Int. J. Lepr. 50(1962)96-101.
12. SHEPARD, C. C. and CHANG, Y. T. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in foot pads of mice. Proc. Soc. Exp. Biol. Med. 109(1962)636-638.
13. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 58(1987)325-337.
14. SUBCOMMITTEE ON CLINICAL TRIALS OF THE CHEMOTHERAPY OF LEPROSY (THELEP) SCIENTIFIC WORKING GROUP OF THE UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME FOR RESEARCH AND TRAINING IN TROPICAL DISEASES. THELEP controlled clinical trials in lepromatous leprosy. Lepr. Rev. 54(1983)167-176.
15. THELEP SCIENTIFIC WORKING GROUP. Report of the First Meeting. Annex 1. Standard protocol for chemotherapy trials in lepromatous leprosy. Geneva: World Health Organization, 1977. TDR / SWG-THELEP/77.3
16. U.S. LEPROSY PANEL (U.S.-JAPAN COOPERATIVE MEDICAL SCIENCE PROGRAM). Spaced clofazimine therapy of lepromatous leprosy. Am. J. Trop. Med. Hyg. 25(1976)437-444.
17. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D.; Institut Marchoux, B.P. 251, Bamako, Mali.
2. D.Sc; Institut Marchoux, B.P. 251, Bamako, Mali.
3. M.D.; Bactériologie et Virologie, Faculté de Médecine Pitie-Salpetriere, 91 Blvd. de l'Hôpital, 75634 Paris 13, France.
Present address for Dr. Husser: Ministère de la Santé Publique et des Affaires Sociales, B.P. 440, N'Djamena, Tchad.
Received for publication on 3 April 1992.
Accepted for publication in revised form on 28 July 1992.