- Volume 60 , Number 4
- Page: 580–6
Patient treatment compliance in leprosy; a critical review
Editorial opinions expressed are those of the writers.
In order to halt transmission of leprosy in the community and to prevent disabilities, the major elements of clinical strategics for treatment and control of the disease are based on the secondary prevention approach, involving early detection and chemotherapy for all types of leprosy.1 Historically, the only measure for control of leprosy was life-long compulsory segregation.2 The discovery of sulfones in the 1940s heralded a new era in the treatment of leprosy.3, 4 Dapsone monotherapy was subsequently used as the standard treatment.2, 5 However, its potential success in the treatment and control of leprosy was constrained by operational and organizational factors.6, 7 The need for prolonged and regular treatment was difficult to achieve owing to poor patient compliance.8 Adverse clinical factors such as primary and secondary dapsone resistance were identified in the late 1960s as a result of giving dapsone alone.9-14 In view of this disastrous clinical situation, combined chemotherapy with more potent drugs was recommended in 1981. Multidrug therapy (MDT) consists of supervised monthly doses and daily self-administration of drugs in a shortened time scale.8 With the introduction of potent drug combinations lor the treatment of leprosy patients, case-detection, treatment delivery and case-holding became of paramount importance. The success of leprosy control programs in the MDT era still depends on operational factors such as patient treatment compliance.15
The purpose of this editorial is to review the past literature on patient treatment compliance in leprosy to determine the incidence of and the factors influencing compliance.
There are two notable reviews 16,17 of investigations in the area of patient treatment compliance in leprosy. Huikeshoven16 summarizes the early history of dapsone in leprosy with special attention to the identification of an optimum dose of dapsone essential to treat leprosy. The problem of drug resistance apparently ended this process and, instead, attention was focused on patient treatment compliance with dapsone administration which directly led to the need for simple methods for detecting sulfoncs in body fluids.
Huikeshoven summarized the results from 12 studies and found that dapsone irregularity was common and, in one study,18 was found to be taken by only 24% of the sample while in another19 it was taken by 87% of the sample. Huikeshoven did not evaluate the studies either individually or as a group, and made no attempt to explain the large differences in the extent of compliance reported. The only abiding similarity between all of the studies reviewed was that compliance was assessed by a physiological measure, namely, the dapsone/creatinine (D/C) ratio method. However, there were significant variations in the original authors' classification of compliance and sample selection strategics. These may have played a significant part in either under- or over-estimating the extent of compliance. The manner in which classification of compliance and sample selection procedures influence the results of compliance investigations in leprosy will be discussed later.
Huikeshoven also presented a limited description of factors known to influence noncompliance. These were mainly concerned with patient expectations and the influence of the paramedical worker on the leprosy patient's compliance behavior. Bijleveld20 found that leprosy patients who ascribed the disease to spirits or witcheraft viewed treatment as temporary relief rather than as a cure. Consequently, regularity of attendance was not a priority for these patients. Varkevisser21 and Huikeshoven and Bijleveld22 also asserted that when paramedical workers were regular in conducting clinics and leprosy patients were treated with respect they turned up for treatment punctually.
Ellard17 also reviewed the findings of previous investigations concerning the regularity of dapsone self-administration and discussed their implications for the strategy of leprosy treatment and control. His review comes to a conclusion similar to Huikeshoven's indicating that irregular self-administration of dapsone is widespread. Ellard discusses the therapeutic implications of poor dapsone compliance and the need for more sensitive methods for monitoring dapsone compliance. However, his review does not attempt to discuss factors that may explain compliance.
The above-mentioned reviews may have been responsible for increasing the awareness of the extent and the importance of compliance in leprosy. Drawing on studies of effective tuberculosis control,23, 24 Ellard also argued for the development of highly effective, supervisable, and intermittent treatment regimens for leprosy which would ensure that the therapeutic outcome was less vulnerable to poor patient compliance. However, Huikeshoven's and Ellard's reviews were more concerned with demonstrating the sensitive physiological methods for monitoring dapsone in body fluids. The methodology of assessing treatment compliance with a biochemical indicator took a leading role in patient compliance research in leprosy. This is useful, but there are other methodological issues such as definition and classification of compliance, research study design, sample selection strategies, and the processes governing compliance which were rarely given due prominence. In order to address these types of limitations in Huikeshoven's and Ellard's reviews of past compliance research in leprosy, a detailed critique of the published research to date will be undertaken here.
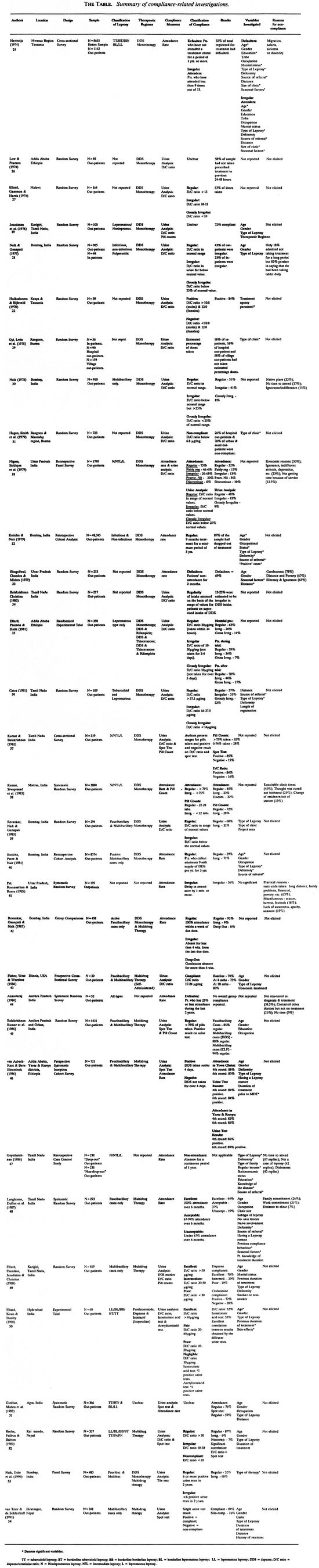
The Table provides an annotated listing of compliance-related investigations in the field of leprosy published since 1974.25-24 The contents of The Table will be discussed as follows: First, various methodological issues will be reviewed followed by an evaluation of the processes governing compliance. Finally, all of the investigations mentioned in The Table will be collectively evaluated, conclusions drawn, and recommendations made for future research.
EXTENT OF COMPLIANCE
The main interest of the investigators in treatment compliance in leprosy was to determine the extent of noncompliance rather than to investigate factors predicting it. In general, between 6%45 and 76%31 of patients failed to comply with prescribed medical regimens in leprosy and, owing to the wide range of dispersion, a mean noncompliance rate is of no value. Higher compliance rates have been consistently reported since the introduction of multidrug therapy (post 1986). This finding is not surprising since patients on MDT are subject to intensive retrieval efforts by leprosy health-care professionals. Better program planning, rapid clinical improvement with the MDT regimens, and the use of aids such as blister calendar packs by some program managers55, 56 have resulted in a significant increase in patient treatment compliance in leprosy.
Another reason for the observed increase in compliance may be due to some patients not being placed on MDT until the treatment agency personnel have established the readiness of the patient to comply with treatment requirements.5 7 The resultant group of patients on MDT is consequently biased in favor of compliers. With one exception,5 4 the policy of some treatment agencies of not placing patients immediately on MDT is not alluded to in the published investigations mentioned in The Table. The exceptional study reports that the current policy of the clinic in which their investigation was based is to give dapsone monotherapy initially and change to MDT after these patients had attended clinic regularly for a few months.
The incidence of compliance reported in The Table may also vary as a result of the different methods of defining, classifying, and assessing compliance.
DEFINITION OF COMPLIANCE
The first step in evaluating any reports of research on treatment compliance is to determine how compliance has been defined by the investigators. Compliance can be defined as the extent to which a person's behavior (in terms of attending clinic, collecting and taking medication) coincides with the advice of the health-care professionals. Treatment compliance investigators in leprosy have generally failed to define compliance, instead they have provided various classifications of compliance.
CLASSIFICATION OF COMPLIANCE
The World Health Organization (WHO) (1980)58recommended that a patient who has attended 75% or more of the treatment sessions be classified as a regular attender. With the introduction of MDT the WHO Study Group (1985)59 defined what they term as "regular treatment" as the receipt of two thirds of the recommended doses of MDT over a specified period. It can be argued that the WHO has defined compliance in a narrow sense, concentrating on attendance in the earlier classification and on the collection of treatment in the latter classification. However, these classification criteria are confined to clinic attendance behavior and do not take into account the individual variability in daily self-administration of the drugs at home. Furthermore, the WHO has not provided a rationale for the basis of their recommendation.60, 61 Many of the investigators mentioned in The Table have not adhered to the WHO recommendations on classifying compilers and, instead, have devised their own classification of compliance.
Generally, two methods are used to classify compliers.62 The first method is a percentage assessment of the degree to which a patient follows a regimen, for example, the percentage of appointments kept out of the total number of appointments made. This method requires that the investigators have knowledge of the denominator (i.e., total number of appointments made). In most leprosy treatment centers the leprosy health-care professional records patients' attendance on their individual medical charts. This method generally has been used by compliance investigators in leprosy to calculate how often and how long a patient attended for treatment.
The second method for classifying compliers is a categorical classification which involves placing patients in an attendance behavioral category rather than simply stating the overall percentage of appointments kept or drug dosages taken. For example, a "good complier" might be a patient who kept at least two thirds of his or her appointments; a "poor complier" might be a patient who kept at least one third of the appointments, and so on. There is a certain arbitrariness in selecting the criteria for inclusion in the various categories. Thus, the "good compliers" to one investigator may be "poor compliers" to another. Evidence of this arbitrariness in classifying compliers can be found by comparing two studies mentioned in The Table. One study38 classifies less than 75% attenders as "irregular"; another study18 classifies 20% to 45% attenders as "irregular" and 46% to 74% attenders as "fairly regular."
This type of variation restricts our ability to compare across studies since uniform criteria for classification of compliance not used, and this method of classification is inexplicit regarding the degree and distribution of compliance rates. Even the same overall rate of attendance at a clinic can conceal a wide range of different patterns of attendance, each with a different implication for understanding the processes involved in compliance. For example, a patient who had failed to attend for the last 4 months in a 12-month period may have left the control area or thought that he had been "cured" whereas another patient who attended intermittently and missed four appointments out of 12 may have had work commitments or simply forgotten to attend. This illustrates that the dynamics that may be operating to make patients compliant to the extent of keeping two thirds of the appointments in a year are probably varied. For this reason, it is important to record the observed overall percentage of attendance and the pattern of attendance behavior, i.e., to use both the percentage and category classification of compliance.
MEASUREMENT OF COMPLIANCE
The way in which compliance is classified influences the type of measurement employed in an investigation. Several types of measures have been used to assess compliance, all of varying accuracy.62-66 These include patient self-reports, physician estimates, pill counts, attendance rates, blood or urine assay, and treatment outcome.67, 68 Compliance investigators in leprosy have usually employed attendance rates, urine analysis, and pill counts as the measures of compliance.
The estimation of compliance is commonly based on a readily and easily measured clement in the regimen, such as attendance rates. The WHO classification of compliance in leprosy is based on the measurement of appointment-keeping, and this has been used by many investigators listed in The Table. This measurement is readily available from patient medical records but it has its limitations. There may be errors and omissions in the records, and there is no guarantee that regular clinic attendance ensures equal regularity in taking medications at home.16, 26
The presence of a drug, its metabolites, or a pharmacologically inert substance can be detected in blood or urine.67 Urine tests have been widely used by investigators to assess patient treatment compliance and are considered to be the most objective and sophisticated measure of compliance.65
The fact that regularity of attendance is no guarantee that leprosy patients take their tablets regularly led to the development of a series of tests to detect dapsone in urine.16 However, a simple behavioral measure of compliance, such as clinic attendance, is an essential first step in the measurement of compliance and is a pre-requisite to the more sophisticated methods of measuring dapsone compliance.
Ellard and his co-workers favor the quantitative urine analysis method (the D/C ratio)27, 35.49, 50, 69 whereas Huikeshoven preferred the qualitative urine analysis method (the modified spot test).70 The great attraction of using a qualitative urine test for monitoring dapsone advocated by Huikeshoven is its cost-effectiveness and simplicity. However, the major disadvantage is that the positivity of urine samples is markedly affected by diuresis. The problems posed by diuresis are virtually overcome in the quantitative D/C ratio method, but it is inevitably a more complicated procedure that takes time and resources. In the investigations where these two types of analysis are used to detect dapsone in urine, the results have been significantly concordant.37, 71
Apart from the discussion concerning which is the best method for monitoring dapsone compliance, there are also differences between investigators in the way they use the results of the tests to categorize compilers and noncompliers. Some investigators use arbitrary cut-off points for sub-classification into various categories of compliance. For example, Ellard and his coworkers in their Malawi study27 classified patients as "irregular" if the urine D/C ratio was between 10 and 15 µg/mg. In their Ethiopian study35 patients were classified as "irregular" if the urine D/C ratio was between 10 and 30 µg/mg. In an investigation conducted in Karigiri, India,49 the leprosy patients were classified as "intermediate cornpliers" if the urine D/C ratio was between 30 to 50 µg/mg and "fair" if the urine D/C ratio was between 20 to 40 µg/mg in another study based in Hyderabad, India.50 These variations in the sub-classification of complices using the results from an identical and a precise measure of compliance makes it impossible to compare the investigations and come to an informed decision concerning the actual extent of compliance.
The failure of compliance investigators in leprosy to agree upon the best method to assess compliance is also coupled with their failure to agree upon a clear definition and classification of compliance based on their favored assessment measure. The arbitrary cut-offs points on the D/C ratio method for sub-classification into various attendance categories by the same investigators, let alone dil Terent ones,36, 43, 52 show that the investigators have paid little attention to the vital methodological issue of classification of compliance and, instead, have often dwelled on finding and arguing for the best method to assess dapsone compliance. Specificity of classification of compliance based on similar measures is essential for replicability of investigations, and this has been absent in the majority of published investigations cited in The Table.
The urine test compliance measure has a disadvantage viz-a-viz the appointmentkeeping measure. If patients are aware of when and why measurements are being obtained, their rate of compliance may be affected at least temporarily. This potential source of bias may be circumvented by obtaining measurements at random and/or at unannounced times in a clinic setting or at the patient's home. In leprosy a major problem with the interpretation of results of dapsone urine tests for measuring compliance is that they do not demonstrate unequivocally whether one or many scheduled daily ingestions of dapsone have been omitted. There seems to be ample evidence from other studies that many patients are quite variable in their compliance patterns, and so a proportion may be mis-categorized depending on the time and day they were tested. 66,72, 73 Alternatively, conducting longterm studies using urine test analyses can be difficult administratively and logistically, and it often requires eliciting repeated or continuous patient cooperation. It was, therefore, not surprising to find that one of the studies50 selected a patient sample that was likely to cooperate over a long period. This strategy directly leads to sample selection bias in favor of compliers.
Multiple random assessments of the same individual, although more costly, should enhance the reliability of urine test measures of compliance and provide clear evidence of the exact magnitude of noncompliance. This has generally been neglected in the past by some investigators.22,51, 54 Evidence in support of the necessity for multiple assessments is provided in a comprehensive study of the self-administration of prothionomide in which over 2000 urine samples were collected from 60 patients over a 2-year period.50 The results of this study show that it is impossible to assess overall compliance on the basis of a single urine test. According to these investigators leprosy patients simply could not be grouped into "good" or "poor" compliers since there was enormous variation in individual patient compliance with a continuous spectrum of drug taking activity.
Pill counts have also been used by some investigators37, 38 in conjunction with other compliance indicators such as urine analysis. Pill counts are easy to carry out and relatively inexpensive in comparison with the urine assays. There are, however, a number of disadvantages associated with this method. Pill counts are usually taken over intervals of several weeks, which does not allow for the assessment of daily variations in compliance. Furthermore, pill counts may be inaccurate since some patients may remove the pills from the container but may not ingest them, and some may even try to sell them if the drugs were given at no cost to them. One study49 reported that 25 of the 44 patients classified on the basis of urine analysis as "poor compliers" were found to have the correct number of capsules and tablets at the pill count. These patients had removed the drugs but had not ingested them.
Therapeutic outcome (subjective assessment by a physician as to whether the disease process has been halted and overcome) is another useful measure of compliance, but it has rarely been used in the investigations reported in The Table. Treatment outcome data, such as remission rates and blood pressure readings, are often included as indirect measures of compliance in some studies in general illnesses.64 This type of measure can be useful in identifying patients who fail to reach treatment goals. Once such patients are identified, more direct measures can be used to determine the degree to which noncompliance has contributed to less than optimal treatment outcome.63
A major disadvantage of using treatment outcome as a measure of compliance is the inexact relationship between compliance and outcome.74 Patients on multiple medications may be compliant with only some medications and yet appear to be clinically improved. For example, in MDT patients may attend a clinic for the supervised dose of rifampin but fail to self-administer clofazimine and dapsone at home50 and, nonetheless, show some clinical improvement. There is also clinical evidence that a high proportion of patients with early paucibacillary leprosy tend to heal spontaneously without any treatment.75, 76
To summarize, therapeutic outcome could be a useful indirect method of assessing compliance in leprosy only when corroborated by other methods of assessing compliance, such as urine assay and attendance counts. Given that each of the measures discussed so far has some disadvantages, the use of a combination of measures may yield a more complete assessment of patient compliance.63,77 In the literature on treatment compliance in leprosy there are some investigations in which two or more indicators of compliance are used.18, 38, 45, 46 Usually the urine analysis measure is used in conjunction with either scheduled appointment-keeping measure or pill counts. These studies report a high correlation between the different measures.
In conclusion, multiple methods of measurement of compliance with treatment regimens in leprosy are essential to determine the true extent of noncompliance. The comment that "accurate measurement of compliance is not easy; easy measurements of compliance are not accurate" is most relevant here.78 Regardless of the measures or combinations of measures used, the definition and classification of compliance is important in terms of specific information obtained from these measures.
RESEARCH DESIGN AND METHODOLOGY
The majority of leprosy compliance investigators used descriptive study design (e.g., single patient group studied at a point in time, and comparisons made between compliers and noncompliers) and a random "grab sample" from a single clinic. Since noncompliance is the central issue, the sample must include all patients who were detected and described treatment for leprosy (including those who quit and dropped out along the way). Patients who had never attended after being detected in a mass survey and those who dropped out of treatment before the sample is selected are likely to have characteristics that distinguish them from other patients.
The investigations reported in The Table have been compromised through failure to follow up all patients who were recommended for or put on treatment at a given point in time. It is only through the accounting for every patient who was present at the inception of treatment, or who was detected during the same period and needing treatment, that the determination of group compliance at a later point in time is meaningful. The systematic loss to analysis of the least compliant patients (those who never kept post-screening referral appointments or those who dropped out entirely after the first few appointments) invalidates the conclusions of a large number of compliance-related investigations in leprosy. It could be argued that the overall range of compliance as reported by a majority of the investigators in leprosy may be considered as over-estimates.
A further criticism of past investigations of patient treatment compliance in leprosy is the researchers' failure to control for sample selection bias. We noted above that the samples of patients on MDT are biased in favor of compliers. Patients whom the investigator regards as noncompliant are excluded from admission, so that the trial is conducted with the group of seemingly cooperative patients who constitute the "compliant sample."79 A pertinent example of this is seen in a study in which the investigators enrolled patients who "had received treatment at the Centre for at least a year, were judged to be likely to continue treatment for at least two years or more, and were willing to allow clinic staif to visit their homes. . ." (p. 164 of50) All of these sample characteristics signify that the research sample selected was biased in favor of compliant patients. We also do not know how the authors were able to "judge" which patients were likely to continue treatment for at least 2 years.
In another compliance investigation49 leprosy patients were enlisted who had been treated for up to 20 years with dapsone monotherapy (mean 1 1 years). These investigators failed to consider the potential bias of their study population with respect to the stage of their disease and its treatment or, indeed, their past level of compliance. Such failures to control for confounding in past investigations on patient treatment compliance in leprosy are ubiquitous.
The other main criticism of the leprosy compliance investigators is their reliance on simple descriptive statistics such as percentages, or simple chi-squared tests on several two-way tables. The classical chisquared approach does not provide estimates of the effects of the variables on each other.80 The process of patient treatment compliance is multifaccted and, consequently, multivariate statistics are essential in determining factors impeding or promoting compliance.
In conclusion, past research on compliance with treatment regimens in leprosy has major shortcomings on methodological grounds, particularly in relation to sample selection. If the study population is biased in any way, this can distort conclusions drawn about compliance.
FACTORS ASSOCIATED WITH COMPLIANCE
The factors investigated and reported in The Tabic could be subsumed under the following headings: patient, disease, and organizational variables.
Owing to the fore-mentioned methodological shortcomings, the results need to be treated with caution and it is inappropriate to draw conclusions by adding together the results of the investigations concerned with different populations and settings, and which used diverse research designs (of varying levels of quality) and different methods of classification and measurement of compliance.
Patient variables. A significant number of studies found no association between age and compliance 19, 28, 31, 40, 43, 45, 46, 48, 49, 51, 52, 54
Two studies25,47 reported an association between education and compliance. Educated patients were significantly more compliant than patients with little or no education. However, another study45 reported no significant association between education and compliance, but this finding has to be treated with caution since the compliance figure was high (94%) and the lack of variance may be responsible for the finding.
A significant number of studies found no association between gender and compliance or occupation and compliance 25, 28, 32, 33, 43, 45, 46, 48, 49, 51, 52, 54
In conclusion, there was no general consistency in the findings between patient socio-demographic variables and compliance with treatment regimens in leprosy.
Disease variables. The associations between disease features of leprosy and subsequent compliance also show conflicting results. A number of investigators found that there was significant association between type of leprosy and compliance 25,32, 36, 40, 47 and a number found no significant association 28, 39, 43, 46, 48-51
The type of leprosy variable (i.e., paucibacillary or multibacillary leprosy) has important theoretical and practical implications. For effective leprosy control, one would expect and demand a higher compliance rate in patients with multibacillary (MB) leprosy since these cases are a prime chemotherapeutic target for interrupting the chain of transmission of leprosy. In addition MB leprosy is the most "severe" in terms of signs and symptoms of the disease, and intuitively one would expect that this may predict compliance. Perhaps the lack of consistency in classification of leprosy used by the investigators may be the reason for nonsignificant findings. For example, Hertroijs25 used TT/BT/BB/BL/LL classification; Cates36 used tuberculoid and lepromatous classification and Gopalkrishnan47 used N/N7L/L classification of leprosy.
The association between deformity and compliance is also conflicting. For example, some investigators found a positive association between the presence of deformity and compliance, while others reported no significant association between the presence of deformity and compliance. Deformity is related to the type of leprosy and the argument advanced above probably applies here.
In conclusion, the type of leprosy and the presence of deformity are important disease-related variables with practical implications. However, their roles in predicting compliance are unclear.
Organizational variables. Source of referral has been found to be significantly associated with compliance.25, 36, 40, 47, 48 Leprosy patients who were self-referred were more compliant than patients who had been detected via mass surveys. The distance to the clinic was not significantly associated with compliance. 25, 36, 51, 54 Only one study33 reported that a long distance to the clinic was significantly related to noncompliance.
Miscellaneous variables. There are a number of miscellaneous factors that have been investigated by a few researchers. For example, seasonal factors have been found to be related to compliance. Hertroijs25 found that more patients failed to comply during the time of reduced agricultural activity when patients tend to visit relatives, and Bhagoliwal, et al.33 found that a significant number of patients failed to comply during the monsoon season. Langhorne, et al.45 found a significant temporal association but could not account for their findings in terms of seasonal migration, climatic features (especially monsoons) or agricultural activity.
The variables of patient knowledge, features of the therapeutic regimen,48 psychosocial factors,47, 48 type of clinic,29, 31 duration of treatment and satisfaction with health-care professionals22 have also been investigated. These findings are too few in number to make any meaningful assessment of their contribution to an understanding of compliance in leprosy.
Finally, the reasons patients give for their noncompliance have been elicited by many investigators,18, 25, 30, 33, 38, 41, 44, 47, 48 but these authors do not provide any conceptual explanations for post hoc categorization of patients' answers. Investigators mention reasons such as "ignorance," "indifferent attitude," "apathy," "not convinced regarding diagnosis," etc., but they have not systematically investigated the role of patients' attitudes in explaining their compliance or noncompliance.
To summarize, this section has reviewed the evidence concerning the relationship of a number of factors to noncompliance with treatment in leprosy. Taking into account different methodological criteria and cultural settings, we can conclude that with the exception of one factor-source of referral-the relationships between the variables investigated and compliance in leprosy is obscure. Patients who have been self-referred for treatment are significantly more likely to be compliant than those patients who have been detected in mass surveys and told to report for treatment.
SUMMARY AND EVALUATION
Previous investigations on patient treatment compliance in leprosy paid little attention to the issue of definition of compliance. The concept of compliance is multifaccted and the processes governing it are also complex. Most investigators did not provide an adequate rationale in their publications for the classification of compliance. Even when an identical measure, such as urine analysis by the D/C ratio method, was employed, the classification varied from one investigator to another even though some investigations were conducted by the same authors.
Previous investigators have paid special attention to the methodology concerning the measurement of dapsone compliance, and innovative techniques have been discovered to detect dapsone in urine. However, this significant advance in the knowledge in measurement of compliance is then compromised by failing to address the methodological issues already stated (i.e., classification) and others, such as research study design and sample selection strategies. Although well-controlled, randomized research designs are necessary in order to provide accurate, generalizable results in the compliance literature, the rigorous sample selection processes made necessary by such designs may cause the results obtained to be under-estimates of the compliance problem. Samples studied in randomized trials must meet entrance criteria that may preclude the exclusion of patients who are not interested in participating in the investigation.
Conversely, compliance may be over-estimated in a number of investigations due to other sample selection problems. Some studies include in their sample only those patients who are willing to participate in a research project. It could be argued that this subgroup of patients may be different in motivational or other characteristics, making them more likely to comply as a group than others who are not willing to participate. Even investigators using the cross-sectional survey method whose sample consisted of all of the patients being treated at a leprosy clinic at a given time did not include.those noncompliant patients who had already begun treatment but immediately discontinued and those who were detected to have leprosy but failed to keep the post-referral appointment.
The high level of compliance found in patients on MDT as opposed to those on dapsone monotherapy is to be expected. In MDT intensive "retrieval" procedures are in operation and only patients who are found to be regular attenders are placed on this type of regimen. Previous investigations on treatment compliance in leprosy have not paid due regard to the confounding effect of the type of treatment variable on subsequent patient compliance behavior.
It should be obvious from the preceding points that the overall range of compliance as estimated in a number of investigations in the leprosy compliance literature could be considered as over-estimated.
The investigation of factors associated with patient treatment compliance in leprosy has been selective. Variables such as socio-demographic and disease features extracted from medical charts are frequently investigated. The statistical analysis of the role these factors play in influencing compliance is also inadequate. This often leads to a conflicting set of results with limited practical value.
The main advantage of using a theoretical orientation in a compliance investigation is that it provides the investigator with a systematic framework for understanding the relevant processes underlying patient treatment compliance. The investigations reported in The Table do not appear to be guided by any theoretical perspective, no post hoc attempt was made to relate the findings to existing theoretical perspectives, and a rationale for including (or excluding) predictor variables were not outlined.
CONCLUSIONS AND RECOMMENDATIONS
Past research on patient treatment compliance in leprosy has been critically evaluated in this editorial. Future research in this area needs to keep several issues in the forefront:
1) A clear and replicable definition and classification of compliance is vital. Wherever possible, both percentage and categorical classification should be used, thus taking into account an individual patient's spectrum of attendance behavior and drugtaking activity.
2) Investigators should utilize direct and indirect measures of compliance, for example, test urine for compliance with prescribed medications, record compliance with scheduled appointment-keeping and report clinical outcome.
3) A prospective inception-cohort study design is essential to record the extent of noncompliance in leprosy. Patients often drop out of treatment early or fail to attend after being positively screened for leprosy. The omission of this group leads to overestimating the magnitude of compliance.
4) The variables to be included for the study of compliance in leprosy should not only include variables such as socio-demographic and disease variables. Compliance is a multifaccted and complex phenomenon, and variables such as family support, patient health beliefs and attitudes, patient satisfaction with the treatment center, etc., are ail important in understanding the process of noncompliance. These variables should form a basis for further enquiry.
5) Statistical analysis should not be restricted to simply reporting percentages and chi-squared tests of association. Multivariate procedures should be used in order to isolate variables that best predict compliance or noncompliance.
6) Wherever possible, investigators should pose questions from a theoretical frame of reference or relate their findings post hoc to existing theoretical perspectives.
These criteria should be helpful to future leprosy compliance investigators in designing studies from which valid conclusions can be drawn. Furthermore, by using these criteria, comparisons between study results will be more informative.
- Atul Vadher, D.Phil.
Department of Experimental Psychology
University of Oxford
South Parks Road
Oxford OX 1 3UD, U.K.
- Mansur Lalljee, D.Phil.
Department of Applied Social Studies and Social Research
University of Oxford
Burnett House
Wellington Square
Oxford OX1 2ER, U.K.
Acknowledgment. We are grateful to Dr. A. Colin McDougall for his helpful comments on earlier drafts of this editorial.
1. WHO Expert Committee on Leprosy. Sixth Report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
2. Rogers, L. Progress towards the eradication of leprosy from the British Commonwealth. RSA J. 102(1954)987-1002.
3. Feldman, W. H., Hinshaw, H. C. and Moses, H. E. The treatment of experimental tuberculosis with promin (sodium salt of p,p'-diamino-diphenylsulfoneN,N'-didextrosc sulfonate); a preliminary report. Proc. Staff. Meet. Mayo Clin. 16(1941)187-193.
4. Faget, G. H., Pogge, R. C, Johansen, F. A., Dinan, J. F., Prcjean, B. M. and Eccles, C. G. The promin treatment of leprosy; a progress report. Public Health Rep. 58(1943)1729-1741.
5. Lowe, J. and Smith, M. The chemotherapy of leprosy in Nigeria with an appendix on glandular fever and exfoliative dermatitis precipitated by sulfones. Int. J. Lepr. 17 (1949) 181-195.
6. Sansarricq. H. Recent changes in leprosy control. Lepr. Rev. Special issue (1983)7-16.
7. WHO Expert Committee on Leprosy. Fifth Report. Geneva: World Health Organization. 1977. Tech. Rep. Ser. 607.
8. WHO Study Group. Chemotherapy of leprosy control programmes; report of a WH O Study Group. Geneva: World Health Organization. 1982. Tech. Rep. Ser. 675.
9. Pettit, J. H. S. and Rees. R. J. W. Sulphone resistance in leprosy: an experimental and clinical study. Lancet 2(1964)673-674.
10. Pettit, J. H., Rees. R. J. W. and Ridley, D. S. Studies on sulphone resistance in leprosy. I. Detection of cases. Int. J. Lepr. 34(1966)375-390.
11. Meade, T. W.. Pearson, J. M. II.. Rees. R. J. W. and North, W. R. S. The epidemiology of sulphoneresistant leprosy, (abstract) Int. J. Lepr. 41(1973)684.
12. Pearson, J. M. H., Rees, R. J. W. and Waters. M. F. R. Sulphone resistance in leprosy. A review of one hundred proven clinical cases. Lancet 2(1975)69-72.
13. Pearson, J. M. H.. Haile G. S.. Barnetson R. S. and Rees, R. J. W. Dapsone-resistant leprosy in Ethiopia. Lepr. Rev. 50(1979)183-199.
14. B.-H. Drug resistance in leprosy-a review. Lepr. Rev. 56(1985)265-278.
15. McDougall, A. C. and Georgicv, G. D. Priorities in leprosy control. Lepr. Rev. 60(1989)1-7.
16. Huikeshoven. H. Patient compliance with dapsone administration in leprosy. (Editorial) Int. J. Lepr. 49(1981)228-258.
17. Etlard, G. A. Drug compliance in the treatment of leprosy. Lepr. Rev. 52(1981)201-213.
18. Nigam, P., Siddique, M. I. A.. Pandey. N. R., Awasthi, K. N. and Sriwastava, R. N. Irregularity of treatment in leprosy patients: its magnitude and causes. Lepr. India 51(1979)521-532.
19. Jesudasan, K., George, C. J. G., Taylor, P. M., Kurian. P. V. and Job, C. K. An evaluation of the selfadministration of DDS in Gudiyatham Taluk. Lepr. India 48 Suppl. (1976)668-676.
20. Bijleveld, I. Leprosy care: patient's expectations and experience. A case study in Western Province, Kenya. Amsterdam: Royal Tropical Institute, 1977.
21. Varkevisser, C. M. Integration of combined leprosy and tuberculoid services within the general health care delivery system Western Province. Kenya. Amsterdam: Royal Tropical Institute. 1977.
22. Huikeshoven, H. and Bijleveld, I. Encouraging results from DDS urine analysis among registered leprosy patients in the Wangas, Kenya; an exception that challenges the rule. Lepr. Rev. 49(1978)47-52.
23. Fox, W. The chemotherapy of pulmonary tuberculosis: a review. Chest 76S(1979)785S-796S.
24. Mitchison, D. A. Treatment of tuberculosis. The Mitchell Lecture, 1979. J. R. Coll. Physicians Loud. 14(1980)91-99.
25. Hertroijs, A. A study of some factors affecting theattendance of patients in a leprosy control scheme. Int.J. Lepr. 42(1974)419-427.
26. Low, S. J. NI. and Pearson, J. M. H. Do leprosy patients take dapsone regularly? Lepr. Rev. 45(1974)218-223.
27. Ellard, G. A., Gammon P. T. and Harris, J. M. The application of urine tests to monitor the regularity of dapsone self-administration. Lepr. Rev. 45(1974)224-234.
28. Naik, S. S. and Ganapati, R. Regularity of dapsone intake by leprosy patients attending urban treatment centre. Lepr. India 49(1977)207-215.
29. Gyi, K. M., Kwin, M. NI., Myaing, Y. Y., Oo, K.M. and Shwe, T. Reliability of dapsone self-administration by leprosy patients in the Rangoon area. Lepr.Rev. 49(1978)283-286.
30. Naik S S Irregularity of dapsone intake in infectious leprosy patients attending an urban treatment centre -its magnitude and causes. Lepr. India 50(1978)45-53.
31. Hagan. K... Smith, S. E., Gyi, K. M., Lwin, M. M., Myaing, Y . Y., Oo, K. M. and Shwe, T. The reliability of self-administration of dapsone by leprosy patients in Burma. Lepr. Rev. 50(1979)201-21 1.
32. Koticha, K. K. and Nair, P. R. R. Treatment defaulters in leprosy: a retrospective study of42,000 cases. Int. J. Lepr. 52(1984)50-55.
33. Bhagoliwal, A., Chandra, J. and Mishra R. S. Some observations on default among leprosy patients. Lepr. India 51(1979)96-102.
34. Balakrishnan. S. and Christian, M. Assessment of self-administration of dapsone in urine. Lepr. Rev. 52(1980)249-251.
35. Ellard, G. A., Pearson, J. M. H. and Haile, G. S. The self-administration of dapsone by leprosy patients in Ethiopia. Lepr. Rev. 52(1981)237-243.
36. Cates. C. J. An assessment of dapsone self-administration in Gudiyantham Taluk. How should urinary' DDS/creatinine ratios be used? Lepr. Rev. 52(1981)55-64.
37. Kumar. A. and Balakrishnan, S. Monitoring the regularity of self administration of dapsone by leprosy patients.'Lepr. India 54(1982)664-670.
38. Kumar, A., Sivaprasad. N.. Anbalagan, M.. Thangavel, M. and Durgambal, K. Utilization of medical agencies and treatment compliance by urban (Madras) leprosy patients. Lepr. India 55(1983)322-332.
39. Revankar, C. R., Naik. S. S. and Ganapati, R. Dapsone compliance in an urban field project. Lepr. India 55(1983)117-121.
40. Koticha. K. K., Patre. B. B. and Nair, P. R. R. Problems of urban leprosy control with special reference to case holding. Int. j. Lepr. 52(1984)482-487.
41. Pal, S., Ramanathan. U. and Ramu. G. A study of the cause of irregularity of patients attending the out-patient department of OIL . Agra. Indian J. Lepr. 57(1985)607-612.
42. Revankar. C. R.. Ganapati, R. and Naik. S. S. Multidrug therapy for paucibacillary leprosy: experience in Bombay. Indian J. Lepr. 57(1985)773-779.
43. Fischer, J. H.. West. D. P. and Worobec, S. M. Evaluation of a continual compliance monitoring program for dapsone in an outpatient Hansen's disease clinic. Int. J. Lepr. 54(1986)517-524.
44. Anandaraj, H. Psycho-social dimensions of drug default in leprosy. Indian J. Lepr. 58(1986)424-430.
45. Balakrishnan. S., Kumar, A., Raja Rao, B. and Patro, T. P. Implementation of tests for monitoring drug compliance of leprosy out-patients under multidrug therapy. Indian J. Lepr. 58(1986)555-559.
46. van Asbeck-Raat, A.-M and Becx-Bleuminck, M. Monitoring dapsone self-administration in a multidrug therapy programme. Lepr. Rev. 57(1986)121-127.
47. Gopalkrishnan. S. Dropouts during treatment for leprosy. Indian J. Lepr. 58(1986)431-440.
48. Langhorne, P., Duflus. P., Berkelely. J. S. and Jesudasan, K. Factors influencing clinic attendance during the multidrug therapy of leprosy. Lepr. Rev. 58(1987)17-30.
49. Ellard, G. A., Pannikar, V. K., Jesudasan, K. and Christian, M. Clofazimine and dapsone compliance in leprosy. Lepr. Rev. 59(1988)205-213.
50. Ellard. G. A., Kiran. K. U. and Stanley, J. N. A. Long-term prothionomide compliance: a study carried out in India using a combined formulation containing compliance among self-motivated leprosy patients. Indian J. Lepr.60(1988)506-509.
51. Girdhar, A. and Mishra, B. Drug compliance among self-motivated leprosy patients. Indian J. Lepr. 60(1988)506-509.
52. Roche, P. W. and Failbus, S. Self administereddrug compliance in Nepali leprosy patients. Trop. Doc. 19(1989)59-61.
53. Naik, S. S., Gole, D. H., Neet, M. R. and Dongre,V. V. Pattern of drug compliance in leprosy patientsattending urban centres-a longitudinal study. IndianJ. Lepr. 62(1990)305-309.
54. van Trier, Y. D. M. and de Soldenhoff, R. Self-administered dapsone compliance of leprosy patientsin eastern Nepal. Lepr. Rev. 62(1991)53-58.
55. Georgiev, G. D. and Kielstrup, R. W. Blister cal-endar packs for the implementation of multidrug ther-apy. Lepr. Rev. 58(1987)249-255.
56. Georgiev, G. D. and McDougall, A. C. Blister cal-endar packs-potential for improvement in the supplyand utilization of multidrug therapy in leprosy controlprogrammes. Int. J. Lepr. 56(1988)603-610.
57. Samy, A. A., Mancheril, J., Manck, K. P. and McDougall, A. C. ALERT-India 1981 - 1989; nine years' experience of leprosy control in the slums of Bombay. Lepr. Rev. 62(1991)315-328.
58. World Health Organization. A Guide to Leprosy Control. Geneva: World Health Organization, 1980.
59. WHO Study Group. Epidemiology of leprosy in relation to control. Geneva: World Health Organization, 1985. Tech. Rep. Ser. 716.
60. Becx-Bleuminck, M. Operational aspects of mul tidrug therapy. (State-of-the art lecture. XIII Int. Lepr. Cong.) Int. J. Lepr. 57(1988)540-551.
61. Huikeshoven, H. Patient compliance in leprosy control: a necessity in old and new regimens. Int. J. Lepr. 53(1985)474-480.
62. Dunbar, J. M. Issues in assessment. In: New Directions in Valient Compliance. Cohen, S. J., ed. Lexington, Massachusetts: D. C. Heath, 1979.
63.Gordis, L. Conceptual and methodologic problems in measuring patient compliance. In: Compliance in Health Care. Haynes, R. B., Taylor, D. L. and Sackett, D. L., eds. Baltimore: Johns Hopkins University Press, 1979.
64. Haynes, R. B. Improving patient compliance: an empirical view. In: Adherence, Compliance and Generalization in Behavioural Medicine. Stuart, R. B., ed. New York: Brunner/Mazel, 1982.
65. McKenncy, J. M. The clinical pharmacy and compliance. In: Compliance in Health Care. Haynes, R. B., Taylor, D. L. and Sackett, D. L., eds. Baltimore: Johns Hopkins University Press, 1979.
66. Roth, H. P. Measurement of compliance. Patient Educ. Counsel. 10(1987)107-116.
67. Epstein, L. H. and Cluss, P. A. A behavioural medicine perspective on adherence to long-term medical regimens. J. Consul. Clin. Psychol. 50(1982)950-971.
68. Morisky, D. E. Nonadherencc to medical recommendations for hypertensive patients: problems and potential solutions. J. Conipl. Health Care 1(1986)5-20.
69. Ellard. G. A.. Gammon P. T., Helmy, H. S. and Rees, R. J. W. Urine tests to monitor the self-administration of dapsone by leprosy patients. Am. J. Trop. Med. Hyg. 23(1974)464-470
70. Huikeshoven, H. A simple urine spot test for mon itoring dapsone self-administration in leprosy treatment. Bull. WHO 64(1986)279-281.
71. Kumar. A. Treatment compliance by leprosy outpatients and its monitoring under field conditions. Lepr. India 56(1984)313-318.
72. Moulding, T. S., Onsad, G. D. and Sbarbaro, J. A. Supervision of out-patient drug therapy with the medication monitor. Ann. Intern. Med. 73(1970)559-564.
73. Roth, H. P. Estimating a patient's cooperation with his regimens. Am. J. Med. Sci. 262(1971)269-273.
74. Eraker, S. A., Kirscht, J. P. and Becker, M. H. Understandingand improving patient compliance. Ann. Intern. Med. 100(1984)258-268.
75. Browne, S. G. Self-healing leprosy: report on 2749 patients. Lepr. Rev. 54(1974)104-111.
76. Jesudasan, K., Bradley, D. and Christian, M. Spontaneous healing in paucibacillary leprosy. Indian J. Med. Res. 81(1985)119-122.
77. Agras, W. S. and Jacob, R. Hypertension. In: Behavioural Medicine: Theory and Practice. Pomerleau, O. F. and Brady, J. P., eds. Baltimore: Williams & Wilkins, 1979.
78. Sackett, D. L. Methods for compliance research. In: Compliance in Health Care. Hayncs, R. B., Taylor, D. L. and Sackett, D. L., eds. Baltimore: Johns Hopkins University Press, 1979.
79. Feinstein, A. R. "Compliance bias" and the interpretation of therapeutic regimen. In: Compliance in Health Care. Haynes, R. B., Taylor, D. L. and Sackett, D. L., eds. Baltimore: Johns Hopkins University Press. 1979.
80. Cox, D. The Analysis of Binary Data. London: Methuen, 1970.
Reprint requests to Dr. Lalljee.