- Volume 60 , Number 4
- Page: 658–9
Suitability of a skin-smear examination needle for leprosy screening by PCR
To the Editor:
Although leprosy has already been eradicated from some parts of the world, the disease still remains a major health problem, especially in developing countries. Recently, some investigators have reported the detection of Mycobacterium leprae, the causative agent of leprosy, by the polymerase chain reaction (PCR) (1-3). We synthesized the PCR primers used in these reported studies, and compared their sensitivity in an attempt to use PCR as a practical screening test for leprosy in regions where the disease is endemic, and where such trials are most needed. The subjects were patients in a leprosy sanatorium in Japan. The needle used for conventional skinsmear examination was used to obtain material from which DNA was extracted. In our experiments, although most patients were negative for acid-fast bacilli in the skinsmear test because all of them had been treated, DNA amplification was observed (The Table). This result demonstrated the sensitivity of PCR, since it was capable of detecting partially digested DNA in dead bacteria from the treated patients.
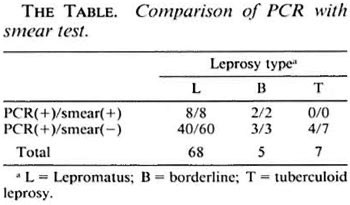
The primers reported by Woods, et al. (3) showed the highest sensitivity, but accuracy of the annealing temperature was essential to prevent nonspecific amplification with human genomic DNA. The accuracy of the temperature setting varies with each PCR processor, and unstable experimental conditions are thought to be likely in developing countries because of variations in electrical voltage and current, which may influence the temperature setting of the processor. Therefore, primers for PCR with higher specificity are desirable for screening tests conducted in the field. Because of the enormous sensitivity of PCR compared with conventional acid-fast staining, M. leprae were even detected from tuberculoid leprosy patients. Although most previous studies used skin-biopsy tissue as the DNA source, our results confirmed the suitability of smear-examination needles for leprosy screening by PCR. The need for further treatment in treated PCR-positive patients will vary from case to case, but positivity in untreated patients means that treatment is necessary in all cases. Using such needles, PCR can become a powerful tool for leprosy screening in endemic regions, allowing treatment to be started at an early stage.
- Yasuyuki Sugita, M.D.
Masamichi Koscki, M.D.
Minora Narita, M.D.
National Sanatorium Tama-Zens/ioen
Tokyo 189, Japan
- Shutaku Kim, M.D.
Norihisa Ishii, M.D.
Hiroshi Nakajima, M.D.
Department of Dermatology
Yokohama City University
Yokohama 236, Japan
REFERENCES
1. HARTSKEERL. R. A., DE WIT, M. Y. L. and KLATSER, P. R. Polymerase chain reaction for the detection of Mycobacterium leprae. J. Gen Microbiol. 135(1989)2357-2364.
2. WILLIAMS, D. L., GILLIS. T. P.. BOOTH, R. J., LOOKER, D. and WATSON. J. D. The use of a specific DNA probe and polymerase chain reaction for the detection of Mycobacterium leprae. J. Infect. Dis. 162(1990)193-200.
3. WOODS. S. A. and COLE, S. T. A rapid method for the detection of potentially viable Mycobacterium leprae in human biopsies: a novel application of PCR. FEMS Microbiol. Lett. 65(1989)305-310.