- Volume 63 , Number 4
- Page: 518–28
CD4+ T-cell responses to recombinant hsp65 and hsp18 of M. leprae and their Trypsin-digested fragments in leprosy: diversity in HLA-DR restriction
ABSTRACT
Mycobacterium leprae heat-shock proteins hsp65 and hspl8 have received immense attention as major T-cell target antigens in leprosy. Both of these hsps and their tryptic fragments were characterized for their ability to stimulate CD4+ T cells derived f rom polar leprosy cases and healthy contacts. The optimal digestion of hsps with trypsin yielded four fragments of hsp65 - TDB65-1 (24 kDa), TDB65-2 (18 kDa), TDB65-3 (17 kDa), TDB65-4 (14 kDa) and three of hspl8-TDB18-l (10 kDa), TDB18-2 (5 kDa), TDB18-3 (3 kDa). While all of these tryptic fragments and undigested hsps triggered CD4+ T cells f rom tuberculoid (TT) leprosy patients and healthy contacts (SI > 2), only two fragments- TDB65-2 and TDB18-3-were found to be stimulatory in anergic lepromatous (LL) leprosy patients (SI = 5.27 and 3.0, respectively). Blocking studies using allele-spccilic anti-DR monoclonal antibodies revealed multiple HLA-Dr restriction, with DR2 providing the strongest restriction in both TT as well as LL leprosy. These findings indicate that M. leprae hsps and their trypsin-digested fragments are promiscuous and recognizable in the context of diverse H LA alleles, of which DR2 is the most efficient restriction element. The 18-kDa fragment of hsp65 and the 3-kDa fragment of hspl8 are the most versatile fragments that could elicit in vitro proliferation in both polar forms of leprosy.RÉSUMÉ
Les protéines de choc de chaleur hsp65 et hspl8 deMycobacterium leprae ont reçu une énorme attention en tant que cibles antigéniques majeures des cellules T dans la lèpre. Ces deux hsp et leurs fragments tryptiques ont été caractérisés quant à leur capacité de stimuler les cellules T CD4+ dérivés de cas de lèpre polaire et de contacts en bonne santé. La digestion optimale des hsp par la trypsine a donné quatre fragments de hsp66 - TDB65-1 (24 kDa), TDB65-2 (18 kDa), TDB65-3 (17 kDa). TDB65-4 (14 kDa)-et trois fragments de hsp 18-TDB18-1 (10 kDa), TDB18-2 (5 kDa), TDB18-3 (3 kDa). Alors que tous ces fragments tryptiques et les hsp non digérés stimulaient les cellules T CD4+ des patients atteints de lèpre tuberculoide (TT) et des contacts en bonne santé (SI > 2), on n'a trouvé que deux fragments-TDB65-2 et TDB18-3-qui étaient stimulateurs chez les patients atteints de lèpre lépromateuse anergique (LL) (SI = 5.27 et 3.0 respectivement). Des études de blocage utilisant des anticorps monoclonaux anti-DR spécifiques pour l'allèle ont révélé une restriction multiple HLA-Dr, avec DR2 produisant la restriction la plus forte dans la lèpre TT comme dans la lèpre LL. Ces découvertes indiquent que les hsp de M. leprae et leurs fragments digérés par la trypsine sont proches et reconnaissables dans le contexte de divers alleles HLA, parmi lesquels DR2 est l'élément de restriction le plus efficient. Le fragment de 18 kDa de hsp65 et le fragment de 3 kDa de hsp 18 sont les fragments aux caractéristiques les plus diverses qui pourraient provoquer une prolifération in vitro dans les deux formes polaires de la lèpre.RESUMEN
Las proteínas de choque térmico hsp65 y hspl8 deMycobacterium leprae han recibido mucha atención como blancos antigénicos reconocidos por las células T en la lepra. Estas hsps y sus fragmentos trípticos fueron caracterizados en cuanto a su capacidad de estimular células TCD4+ obtenidas de casos polares de la lepra y de individuos sanos. La digestión óptima de las hsps con tripsina produjo 4 fragmentos de la hsp65 (TDB65-1 de 24 kDa, TDB65-2 de 18 kDa, TDB65-3 de 17 kDa, y TDB65-4 de 14 kDa), y 3 fragmentos de la hspl8 (TDB18-1 de 10 kDa, TDB18-2 de 5 kDa. y TDB18-3 de 3 kDa). Todos los fragmentos trípticos y las proteínas no digeridas estimularon a las células TCD4+ de los pacientes con lepra tuberculoide (TT) y de los contactos sanos, pero sólo 2 de los fragmentos (TDB65-2 y TDB18-3) fueron estimulatorios para las células de los pacientes lepromatosos, LL (SI= 5.27 y 3.0, respectivamente). Los estudios de bloqueo con anticuerpos monoclonales anti-DR, alelo específicos, revelaron restricción múltiple, siendo DR2 el responsable de la restricción más fuerte tanto en los pacientes TT como en los LL. Los hallazgos indican que las hsps de M. leprae son promiscuas y son reconocidas en asociación con diversos alelos HLA entre los cuales DR2 es el elemento de restricción más eficiente. Los fragmentos de 18 kDa de hsp65 y de 3 kDa de hspl8, fueron los más versátiles en cuanto a que pudieron inducir la proliferación in vitro de los linfocitos en ambas formas polares de la lepra.Protective immunity, immmunopathology and the clinical manifestations of leprosy are dependent on the generation of antigen-specific T cells against Mycobacterium leprae (4,10,13,17).Over the past few years, a large number of M. leprae antigens have been characterized (5, 33, 35), including several heat-shock proteins (hsps) with strong immunogenicity (34). In particular M. lep rae-derived hsp65 and hsp 18 have been well documented as immunodominant antigens for both T and B cells of healthy as well as mycobacterial disease-affected individuals in the context of diverse HLA class II molecules (7,8,10,11,22,23). In recent years, a number of studies have been conducted to define T-cell determinants on both of these stress proteins (14, 23, 28, 31). Since CD4+ T cells constitute early crucial elements of immune recognition (3), the delineation of epitopes on M. leprae hsps triggering CD4 + cells of the right type is important in order to understand the immunopathobiology of leprosy and to design future strategies for its control.
In the present study, we have attempted to delineate hsp65 and hsp18 for their potential CD4+ T-cell immunogenicity in tuberculoid (TT) leprosy, lepromatous (LL) leprosy and healthy contacts. An effort was made to define these responses using tryptic digests of hsp65 and hsp 18 and the HLA-DR restriction pattern of polyclonal T cells derived from patients with polar leprosy. We identified specific antigenic fragments of M. leprae hsps that could elicit CD4 + T-cell proliferative responses which were restricted by diverse HLA-DR molecules in both polar forms of leprosy.
MATERIALS AND METHODS
Subjects
Patients and control subjects were obtained from the outdoor clinic of the School of Tropical Medicine, Calcutta, India. Nineteen TT and 18 LL leprosy patients diagnosed on clinical and bacteriological criteria and classified according to Ridley and Jopling (24) were included in the study. The diagnosis was confirmed by histopathology in each case. All patients were diagnosed within 6 months prior to their inclusion in the study, and none had received any form of steroids during their antileprosy therapy. The control group consisted of seven healthy individuals from the same endemic area living in close contact with leprosy patients.
Antigens and antibodies
Affinity chromatography purified recombinant hsp65 and hsp 18 of M. leprae were generous gifts from Dr. J. D. A. van Embden, National Institute of Public Health and Environmental Protection, Bilthoven, The Netherlands, through the WHO-IMMLEP program. Anti-CD4 and anti-CD8 cloned hybridomas were kindly provided by Dr. Santu Bandyopadhaya, Indian Institute of Chemical Biology, Calcutta, India. The culture supernatants of these hybridoma cell lines at a 1:20 dilution were used as the sources of anti-CD4 and anti-CD8 monoclonal antibodies.
Trypsin digestion of M. leprae hsps
hsp65 and hspl8 [120 µg in 60 µl of 10 mM phosphate buffered saline (PBS)] were individually incubated at 37ºC with 3 µl (0.041 units) of trypsin (Sigma Chemical Co., St. Louis, Missouri, U.S.A., 1.5 µg/ml) for precisely optimized time, i.e., 1 min for hsp65 and 2.5 min for hspl8. The reaction was stopped by the addition of 40 µl of gel loading buffer (50 mM Tris, 10 mM dithiothreitol, 2% SDS, 0.1% bromophenol blue, 10% glycerol) and heating in boiling water for 3 min. Digested hsps were electrophoresed on a 15%-20% gradient SDS-PAGE gel in order to separate the trypsindigested peptide fragments according to their molecular weights. Separated bands were transferred onto the nitrocellulose paper (29) and processed further for T-cell Western blot analysis (1).
HLA typing
HLA typing of all patients and controls was carried out by the standard microlymphocytoxicity test (27). The class II antigens (HLA-DR and DQ) were determined using nylon wool purified B lymphocytes on a set of sera defining DR1 to DR16 and DQ1 to DQ9 specificities. The serological typing was subsequently confirmed by polymerase chain reaction (PCR)-oligotyping using biotinylated probes (19).
Standardization of T-cell proliferation assay
The modified MTT assay system [3-(4, 5dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide; Loba Chemie, Bombay, India] was employed with various initial numbers of cells/well and various doses of PHA (20, 26). The tctrazolium ring is cleaved by mitochondrial dehydrogenase of viable cells leading to the formation of dark-violet formazan crystals, which can be dissolved in acid-SDS (10% SDS in 0.0 IN HC1) by overnight incubation at 37ºC. The color intensity of the crystal solutes are proportional to the cell number and can be read on a micro ELISA reader using a wavelength of 590 nM. The dose-dependent mitogenic responses were proportional to the initial cell concentration of the well. The cell concentration of 2 x 105 cells/well was found to be optimal in the assay system and was used in all subsequent experiments.
CD4+ T-cell proliferation
From each individual to be tested, 20 ml of heparinized blood was collected under aseptic conditions and peripheral blood mononuclear cells were isolated by Ficollhypaque gradient centrifugation method. Monocytes were allowed to adhere to the bottom of the 96-well flat-bottom plate by incubation for 2 hr in a fully humidified 5% C02-air mixture at 37ºC and the nonadherent cells (T and B cells) were separated. From the pool of nonadherent cells, B lymphocytes and CD8 + T cells were selectively removed by complement mediated lysis (l5) using anti-IgM antisera and anti-CD8 monoclonal antibodies. The purity of the CD4+ T cells was checked by indirect immunofluorescence and found to be > 90%. Purified CD4+ T cells thus obtained (2 x105/well) were co-cultured with adhered autologous monocytes in triplicate in RPMI1640 containing FCS (10%), L-glutamine (2 mM), gentamycin (50 µg/ml ) in the presence of hsp65 (5 mg/ml)/or hspl8 (7.5 mg/ml)/ or their tryptic fragments in varying doses ( µl /ml). Positive controls with PHA (5 µg/ ml) and negative controls without any mitogen were set up in parallel with each experiment. After 5 days of incubation at 37ºC in a humidified environment with 5% CO2 the cell proliferation was measured by the modified MTT method (20, 26).
The results are expressed as a stimulation index [SI = Optical density (OD) of the culture with antigen/OD of culture without antigen at 590 nM]. A mean SI of > 2 was considered as indicative of cell proliferation.
Inhibition of T-cell response by allele-specific anti-DR Mabs
An inhibition assay was performed by blocking one of the DR molecules on the heterozygous monocytes by the addition of allele-specific anti-DR monoclonal antibodies (M/s Pel-Freez, Rogers, Arkansas, U.S.A.) to the adherent monocytes at least half an hour before the addition of purified CD4+ T cells and the antigens. Most of these antibodies inhibited the proliferation (compared to that of uninhibited analogous cultures) in a dose-dependent manner and, in most cases, were used at a 1:50 dilution which was found to be optimal. The percent inhibition was calculated as follows: 1 - (OD of Mab added culture/OD of Mab free culture at 590 nM) x 100.
Statistical analysis
The cell proliferation data were expressed as the mean SI ± standard error of the mean (S.E.M.) and the inhibition data as the percent inhibition ± S.E.M. The statistical significance for the cell proliferation experiment among various patient groups in response to different antigens was calculated by nonparametric methods: Kruskal-Wallis and Wilcoxon rank sum tests (6). Statistical significance for the data on inhibition of cell proliferation was determined by Student's t test.
RESULTS
Trypsin digestion of M. leprae hsps
Digestion of hsp65 for 1 min generated four peptide fragments referred to as TDB65-1 (24 kDa), TDB65-2 (18 kDa), TDB65-3 (17 kDa) and TDB65-4 (14 kDa). hsp 18, on the other hand, yielded three fragments at optimal digestion for 2.5 min: TDB18-1 (10 kDa), TDB18-2 (5 kDa) and TDB18-3 (3 kDa) (Fig. 1 a and b).
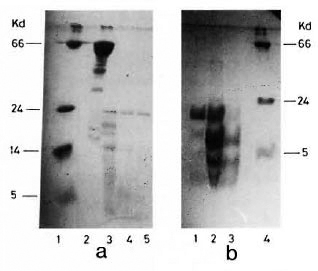
Fig. 1. SDS-PAGE gel stained with Coomassie blue RL50 showing trypsin digested fragments of M. leprae -derived hsps. a = hsp65 with varying digestion times,1 min (lane 2), 2.5 min (lane 3) and 5 min (lane 4). Lane 3 shows four fragments, TDB65-l (24 kDa), TDB65-2 (18 kDa), TDB65-3 (17 kDa) and TDB65-4(14 kDa). Lane 1 was loaded with molecular weight markers. b = hsp18 digested for 1 min (lane 1), 2.5 min (lane 2) and 5 mins (lane 3). Lane 2 shows three fragments, TDB18-1 (10 kDa), TDB18-2 (5 kDa) and TDB18-3 (3 kDa). Lane 4 was loaded with molecular weight markers. Topmost band represents undigested HSP18.
CD4+ T-cell responses to hsp65 and its tryptic fragments
hsp65 was found to be recognized by CD4 + T cells obtained from healthy contacts (mean SI = 5.8) and patients with TT leprosy (mean SI = 5.2); it failed to stimulate cells from LL leprosy subjects (SI = 1.6, p < 0.001). Similar responses were observed with TDB65-1 in TT patients and healthy contacts as compared to LL patients (p < 0.001) with the proliferation magnitude as found in the undigested hsp65. Significant reactivity also was found against TDB65-3 and TDB65-4 in TT patients compared to that of LL patients (p < 0.001). However, reactivity of these two fragments was of a much reduced magnitude than thatobtained with either the whole hsp65 or its first two tryptic fragments, TDB65-1 and TDB65-2 (Fig. 2).
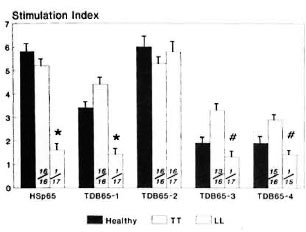
Fig. 2. CD4+ T-cell proliferative responses expressed as mean stimulation index (bar) ± S.E.M. (vertical line at top of bar) of patients with leprosy and healthy contacts against hsp65 and its four tryptic fragments. Numbers at bottom of each bar indicate number of responders/number of individuals tested; p values: * = < 0.001 (LL leprosy vs healthy contacts/orTT leprosy); # = < 0.001 (LL leprosy vs TT leprosy).
Contrarily, TDB65-2 was not only found to be immunogenic in TT patients (mean SI = 5.3) and healthy contacts (mean 6.0), but also in LL patients (mean SI = 5.8). This was the only tryptic fragment of hsp65 that could bypass its specific unresponsiveness in lepromatous leprosy.
CD4+ T-cell responses to hspl8 and its tryptic fragments
hspl8 was found to be immunogenic in TT patients and healthy contacts, but not in LL patients (p < 0.001). Its larger two fragments, TDB18-1 and TDB18-2, showed reactivity of a reduced magnitude in both TT patients as well as healthy contacts (SI > 2.0) but LL patients remained unresponsive (SI < 2.0). However, the SI values between individual groups were not statistically significant in relation to these two fragments. On the other hand, TDB18-3 was found to be potentially immunogenic in all three groups studied, with significant SI values of 6.8, 5.4 and 3.1, respectively. Although this 3-kDa fragment stimulated CD4+ T cells from anergic LL patients (SI = 3.1), the response was significantly lower compared to TT patients and healthy contacts (p < 0.01) (Fig. 3).
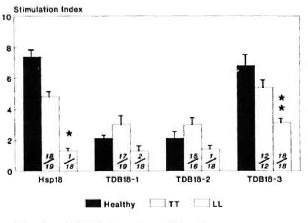
Fig. 3. CD4+ T-cell proliferative responses expressed as mean stimulation index (bar) ± S.E.M. (vertical line at top of bar) of patients with leprosy andhealthy contacts against hsp18 and its tryptic fragments. Numbers provided at bottom of each bar denote number of responders/number of individuals tested; p values: * = < 0.001; ** = < 0.01 (LL leprosy vshealthy contacts/or TT leprosy).
HLA-DR restriction
The inhibition of the T-cell responses by allele-specific anti-DR monoclonal antibodies (Mab) against each of the antigens tested was expressed as a percent inhibition. A > 50% inhibition was considered significant. The data thus obtained reflects the magnitude of restriction imposed by specific HLA-DR alleles.
DR restriction of hsp65 and its fragments
The proliferative response to undigested hsp65 was significantly inhibited (> 50%) by anti-DR2 Mab in 14 out of the 19 DR2positive TT patients (Fig. 4a). Although similar inhibition was observed with several other non-DR2 molecules (e.g., DR1, DR5 and DR7), it was significantly higher when anti-DR2 Mab was used (p < 0.001). For example, in TT patients carrying DR1/ DR2 phenotypes (N = 7), the mean inhi bition by anti-DR 1 was low (18.5%); whereas in one patient carrying DR1 along with a non-DR2 allele, it was found to be appreciably higher (68.2%). Similarly, anti-DR5 inhibited the response by 72.9% in a DR5/non-DR2 patient but in a DR5/DR2 positive patient, the inhibition was only 18.2%, suggesting stronger immunogenic affinity of DR2 for hsp65 and competition imposed by it on other DR alleles.
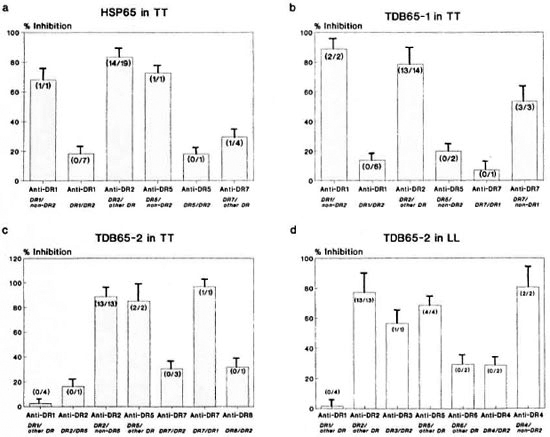
Fig. 4. Percent inhibition of CD4+ T-cell proliferative responses of leprosy patients using allele-specific anti-DR Mabs against: a = HSP65 in TT; b = TDB65- l in TT; c = TDB65-2 in TT; d = TDB65-2 in LL leprosy. Each bar represents mean percent inhibition; vertical line at top of bar denotes S.E.M. Numbers in parenthesesindicate number of individuals showing > 50% inhibition/number of individuals tested.
TDB65-1. Responses to the TDB65-1 fragment in TT patients showed stronger inhibition by blocking of HLA-DR2 in comparison with all other non-DR2 alleles reaching the significance level (p < 0.001). In six patients with the DR1/DR2 phenotype, the inhibition by anti-DR 1 was less (13.8%) but when DR1 is associated with a non-DR2 allele (N = 2), the inhibition by anti-DR 1 was found to be very high (88.9%). Similarly, inhibition by anti-DR7 was low in a patient with a DR7/DR1 phenotypc (7.1%) but the same was quite high (53.6%) in the DR7/non-DRl phenotypc (N = 3). The response to this fragment could also be inhibited by anti-DR5 in DR5/non-DR2positive patients. Thus, TDB65-1 was found to be immunogenic in the context of DR2, DR1, DR7 and DR5, with DR2 providing the strongest restriction (Fig. 4b).
TDB65-2. The response to this unique fragment in TT patients was inhibited significantly by the blocking of DR2 (88.8%), more so than all other non-DR2 alleles (p < 0.01). Inhibition by anti-DR5 in two patients carrying DR5/DR2 or DR1 was high (85%), suggesting that DR5 is also a strong presenter of this fragment. Anti-DR7 inhibited the response in one patient with a DR7/DR1 phenotype (96.9%). However, in the presence of DR2 in a DR7-positive phenotype (N = 3) inhibition by anti-DR7 was found to be low, suggesting that DR2 can compete with DR7 for immunogenic presentation of this fragment. Only one DR8positive patient who carries DR2 as the other phenotype was available and the TDB652-specific response in this patient could be inhibited (32%) by anti-DR8 Mab (Fig. 4c).
In DR2-positive LL patients (N = 13), the CD4+ T-cell proliferative response to this fragment was inhibited strongly by anti-DR2 Mab as compared to that obtained by the blocking of other DR alleles (77.2%, p < 0.01). However, the response could also be inhibited by anti-DR5 (68.4%), anti-DR3 (56.6%) and anti-DR6 (29.4%) Mab (Fig. 4d). In DR4-positive subjects, inhibition by anti-DR4 in patients (N = 2) bearing DR4/ non-DR2 alleles was high (80.5%), although the presence of DR2 led to a drop in the response to 28.6%. This suggests that DR2 is the strongest restriction allele for TDB65-2 in LL patients also and competes with DR4 for immunogenic binding.
TDB65-3. The CD4+ T-cell response to this 17-kDa fragment was strongly inhibited (82.1%) by anti-DRl in TT patients (N = 9) compared to non-DRl antibodies (p < 0.01). Interestingly, inhibition by anti-DR2 was very low (9.2%, N = 9) compared to that by anti-DRl (p < 0.001). In a DR7/ DR1-positive patient, inhibition by anti-DR7 was low (8.6%) compared to the DR7/ non-DR 1 patients (N = 3) in whom the mean inhibition was 92.9%. Generally, anti-DR7 inhibited the response more strongly than anti-DR2 (p < 0.01).
HLA-DR restriction for the TDB65-4 fragment could not be determined due to certain unavoidable constraints such as contamination of the stock antigen.
DR restriction of hspl8 and its fragments
The CD4+ T-cell response to hspl8 was studied in 19 TT patients. The mean inhibition by anti-DR2 was significantly higher (86.3%) as compared to other non-DR2 antibodies (p < 0.1), and all patients gave inhibition levels of > 50%. Inhibition by anti-DR 1 (N = 4) and anti-DR5 (N = 1) was found to be 14.2% and 1.6%, respectively, which is significantly lower than that obtained by anti-DR2 (anti-DR 1, p < 0.001; anti-DR5, p < 0.01) (Fig. 5a). Inhibition by anti-DR7 and anti-DR8 followed two distinct patterns. In the presence of DR2, the inhibition by antibodies to these alleles was low (26.7% and 20%, respectively). On the other hand, in the absence of DR2, the inhibition by anti-DR7 (N = 1) and anti-DR8 (N = 3) was much higher (96% and 97% respectively). Since the number of patients with all of these combinations was small, statistical calculations could not be done for these alleles.
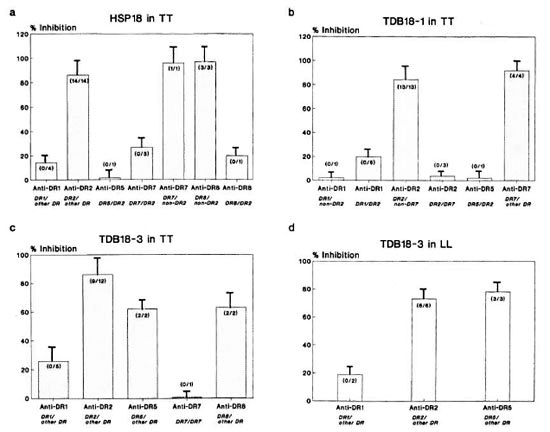
Fig. 5. Percent inhibition of CD4+ T-cell proliferative responses of leprosy patients using allele-specificanti-DR Mabs against: a = HSP18 in TT; b = TDB18-1 in TT; c = TDB18-3 in TT; d = TDB18-3 in LLpatients. Each bar represents mean percent inhibition; vertical line at top of bar denotes S.E.M. Numbers inparentheses denote number of individuals showing < 50% inhibition/number of individuals tested.
TDB18-1. Using TDB18-1, maximum inhibition was provided by the blocking of DR7 (91.8%) which was much higher compared to the blocking of non-DR7 alleles (p < 0.05). Minimum inhibition was obtained with anti-DR5 in one patient (2%). Anti-DR2 also showed strong inhibition, but only in the absence of DR7 in the phenotypc. On the contrary, the presence of DR7 in DR2positive TT patients rendered anti-DR2 a poor inhibitor of the response (p < 0.001). When DR7 was associated with DR1, only 2% of the response could be inhibited by anti-DRl, but in the absence of DR7 in DR1-positive patients, the anti-DRl-mediated inhibition was 19.7% (N = 6). Thus, TDB18-1 is a multideterminant cluster peptide of hspl8 of M. leprae (Fig. 5b).
TDB18-3. In 9 out of 12 TT patients tested, anti-DR2 was found to be a strong inhibitor of polyclonal T-cell response to the versatile fragment TDB 18-3 (86.%) as compared to blocking other non-DR2 alleles (p < 0.01) (Fig. 5c). This anti-DR2mediated inhibition also was significant when compared with other individual anti-DR antibodies, such as anti-DRl (p < 0.01), anti-DR8 (p < 0.001) and anti-DR5 (p < 0.01)
In LL patients (N = 8) on the other hand, anti-DR5 was found to be the strongest inhibitor (78%, N = 3) followed by anti-DR2 (72.9%, N = 6) and anti-DRl (19%, N = 2) (Fig. 5d). Thus, this unique fragment was restricted by the maximum number of HLA-DR alleles, namely, DR2, DR5, DR8, and DR1 not only in TT patients but also in anergic LL cases.
DISCUSSION
In order to develop effective immunomodulators odulators for leprosy, it is important to define the antigenic sites on M. leprae seen by the CD4+ T cells that constitute early and crucial events in effector immune function. In the present study, we report in vitro proliferative responses of CD4+ T cells from polar leprosy patients against M. leprae-de rived hsp65, hspl8 and their tryptic fragments. In their native form, both hsp65 and hspl8 were found to be immunoreactive in TT leprosy patients and healthy contacts, but failed to induce proliferation in LL subjects. The unresponsiveness observed in LL patients may be attributed to suppressor mechanisms directed against these hsps (32). Since the reactive subjects in this study (both TT patients and healthy contacts) were from an outbred population, it may be envisaged that these proteins bear multiple T-cell epitopes with affinity for various HLA class II alleles. The reduced magnitude of T-cell reactivity against 17-kDa (TDB65-3) and 14-kDa (TDB65-4) fragments suggests that these might have lost some of the potential epitopes present on the intact hsp65 molecule. Interestingly, TDB65-2 triggered polyclonal CD4+ T cells derived not only from TT patients and healthy contacts but also from LL patients, suggesting that this 18-kDa fragment of hsp65 could bypass the selective T-cell anergy in LL leprosy. The magnitude of responses against this fragment was found to be similar in all three groups studied, suggesting that most of the helper/inducer epitope(s) of the intact molecule are clustered in this 18-kDa fragment without any suppressogenic element.
Regarding hspl8, although the two big fragments (10 kDa and 5 kDa) were less immunogenic in TT leprosy and healthy contacts, the smaller 3-kDa fragment appeared to have immunogenicity similar to the whole hspl8 in the same group of subjects. Further, this 3-kDa fragment was immunoreactive even in LL patients, suggesting that this fragment could circumvent the suppressor mechanism(s) directed against hspl8 or its two other tryptic fragments. The possibility of revealing "cryptic" epitopes or the effect of competition can also be considered for the observed universal immunoreactivity of the 3-kDa fragment of hspl8. Thus, it is clear that at least one fragment in each on the two major stress proteins, namely, TDB65-2 (18 kDa) and TDB 18-3 (3 kDa) escapes the immunological unresponsiveness of LL leprosy possibly due to the deletion of the suppressogenic elements present on intact hsps.
For the development of a potential vaccine for leprosy in the outbred human population, it is necessary for a candidate molecule to be presented by multiple HLA class II alleles (2,12). The data in the present study revealed that while hsp65 can bind immunogenically with a battery of DR molecules (i.e, DR2, DR 1, DR5 and DR7), HLA-DR2 appears to be the strongest presenting allele. Recently, Mutis and co-workers (21) have demonstrated that an epitope that maps between the amino acid residues 439 and 448 of the mycobacterial hsp65 is restricted/presented by HLA-DR2 (DRB1*1503) in the Surinam black population. None of the paticnts/controls in the present study had DRI31*1503. In fact, this unique subtype of DR2 is characteristic of negroids and is totally absent in Asian Indians (18). Further, DR3 restriction earlier reported in TT leprosy (9) could not be detected in our patient group. Instead, we obtained evidence for the presence of DR7-restricted determinants on hsp65 perhaps for the first time in this sample of Asian Indian patients. Similarly, DR2 was found to be the strongest presenter of the 18-kDa fragment in both polar forms of leprosy. Although, the restriction imposed on TDB65-2 by DR2 was almost similar in TT and LL leprosy patients, that by other DR alleles was varied in the two patient groups, i.e, DR1, DR5, DR7 in TT leprosy and DR3, DR4, DR6 in LL leprosy. In this respect, this fragment appears to contain multiple epitopes with affinity for several DR alleles. Interestingly, DR8 restriction was found only for this fragment in TT leprosy and not for any other fragment tested, including the whole molecule. It is possible that DR8 restricted epitope(s) may be cryptic in nature, inaccessible for immunogenic presentation and expressed only on the 18kDa fragment of hsp65 after trypsin digestion. Thus, de novo trypsin digestion widens the DR restriction of hsp65.
The results on hspl8 revealed multiple DR restriction with DR2 as the strongest restricting element in TT patients. Among its tryptic fragments, the 10-kDa fragment (TDB18-1) was restricted by multiple DR alleles (DR7, DR1, DR2, DR5) with DR7 being the potential antigen-presenting allele. Interestingly, the smallest of the three fragments, TDB18-3, stimulated CD4+ T cells derived from both TT as well as LL patients and was restricted by multiple DR alleles. For this fragment, the highest restriction was provided by DR2, while DR7 restriction was close to zero. This indicates that DR7-restricted determinant(s) are expressed strongly on TDB18-1, and they are either absent or not properly oriented for immunogenic presentation on TDB18-3.
The present study provides evidence that hsp65, hspl8 and their tryptic digests are ubiquitous so far as their immunogenic affinity for HLA-DR specificities is concerned. Further, an 18-kDa fragment of hsp65 and a 3-kDa fragment of hspl8 are most versatile and potentially immunogenic in both polar forms of leprosy. The study provides evidence that multiple DR alleles are involved in MHC restriction to M. leprae hsp antigens. The DR alleles associated with the highest mean inhibition are those with the highest affinities for relevant peptides. Among these, DR2 is the strongest restricting allele for the mycobacterial antigens tested in this experiment. This possibly reflects the dominance of DR2-restricted helper T-cell reactivity in the TT or reactive form of leprosy. Indeed, DR2 has been reported to be strongly associated with TT leprosy (16,30) and pulmonary tuberculosis (25) in the Indian population. Our results of dominant DR2 restriction of M. leprae hsps in immunoreactive forms of leprosy explain the immunological basis for the observed DR2 association in polar TT leprosy.
It is highly probable that these tryptic fragments may carry multiple epitopes, so that a diverse HLA-DR restriction is possible. Further fragmentation of these tryptic digests and delineation of their amino acid sequences could provide valuable information in defining the critical determinants in the M. leprae hsps. In this respect, although our observations are preliminary, the knowledge about these M. leprae hsps and their DR restriction pattern is important for designing effective immune intervention strategies in leprosy.
Acknowledgment. The authors thank the Reagent Reference Center of the National Institute of Immunology, New Delhi, India, for providing antihuman IgM and other antisera. The authors also are grateful to the late Prof. S. Chaudhury, School of Tropical Medicine, Calcutta, India, for providing valuable clinical material and to Mr. Shiv Shankar Dayal for preparing the manuscript. The study was supported by grants received from the Indian Council of Medical Research (ICMR) and the Department of Biotechnology (DBT), Government of India. A CSIR Fellowship (Government of India) to D. K. Mitra is gratefully acknowledged.
REFERENCES
1. ABOU-ZEID, C, FRILLEY, E., STEELE, J. and ROOK, G. A. W. A simple new method for using antigens separated by Polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J. Immunol. Meth. 98 (1987) 5-10.
2. BERZOFSKY, J. A. Development of artificial vaccines against HIV using defined epitopes. FASEB J. 5 (1991) 2412-2418.
3. BERZOFSKY, J. A., BRETT, S. J., STREICHER, H. Z.and TAKAHASHI, H. Antigen processing for presentation to T-lymphocytes: function, mechanisms and implications for T cell repertoire. Immunol. Rev. 106 (1988) 5-31.
4. BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80 ( 1984) 5-28.
5. BRITTON, W. J., HELLQVIST, L., BASTEN, A. and RAISON, R. L. Mycobacterium leprae antigens involved in human immune responses. I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135 (1985) 4171-4177.
6. CONOVER, W. J. Practical Non-Parametric Statistics. New York: John Wiley and Sons, 1980, pp. 213-239.
7. DE VRIES, R. R. P., OTTENHOFF, T. H. M., LI, S.-G.and YOUNG, R. A. HLA class II restricted helper and suppressor clones reactive with Mycobacterium leprae. Lepr. Rev. 57 Suppl. 2 (1986) 113-121.
8. DOCKRELL, H. M., STOKER, N. G., LEE, S. P., JACKSON, M., GRANT, K. A., JOUY, N. E., LUCAS, S. B., HASAN, R., HUSSAIN, R. and MCADAM, K. P. W. J. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57 (1989) 1979-1983.
9. HAANEN, J. B. A. G., OTTENHOFF, T. H. M., LAI A FAT, R. F. M., SOEBONE, H., SPITS, H. and DEVRIES, R. R. P. Mycobacterium /eprae-specific T cells from a tuberculoid leprosy patient suppress HLA-DR3-restricted T cell responses to an immunodominant epitope on 65-kDa hsp of mycobacteria. J. Immunol. 145 (1990) 3898-3904.
10. HUNTER, S. W., RIVOIRE, B., MEHRA, V., BLOOM, B. R. and BRENNAN, P. J. The major native proteins of the leprosy bacillus. J. Biol. Chem. 265 (1990) 14065-14068.
11. KAUFMANN, S. H. E. Heat shock proteins and pathogenesis of bacterial infections. Springer Semin. Immunopathol. 13 (1991) 25-36. (50 refs)
12. KlLGUS, J., ROMAGNOLI, P., GUTTINGER, M., STUBER, D.,ADORINI, L. and SINIGAGLIA, F. Vaccine T-cell epitope selection by a peptide competition assay. Proc. Natl. Acad. Sci. U.S.A. 86 (1989) 1629-1633.
13. LAGRANGE, P. H. Cell mediated immunity anddelayed-typc hypersensitivity. In: The Mycobacteria-A Source Book. Part B. Kubica, G. P. and Lawrence, G. W., eds. New York: Marcel Dekker, 1984, pp. 681-720.
14. LAMB, J. R., IVANYI, J., REES, A. D. M., ROTHBARD, J. B., HOWLAND, K., YOUNG, R. A. and YOUNG, D. B. Mapping of T-cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 6 (1987) 1245-1249.
15. MASON, D. W., PENHALF, W. J. and SEDGWIK, J. D. Separation of lymphocyte by complement mediated lysis. In: Lymphocyte and Practical Approach. Klaus, G. G. B., ed. London: IRL Press, 1987, pp. 50-51.
16. MEHRA, N. K. Role of HLA linked factors in governing susceptibility to leprosy and tuberculosis. Trop. Med. Parasitol. 41 (1990) 352-354.
17. MEHRA, N. K., DASGUPTA, A. and VAIDYA, M. C. An evaluation of the immune state in leprosy. Lepr. India 48 (1976) 231-237.
18. MEHRA, N. K., RAJALINGAM, R. and GIPHART, M. J. Generation of DR51-associated DQA1, DQB1 haplotypes in Asian Indians. Tissue Antigens 46 (1995) in press.
19. MEHRA, N. K., RAJALINGAM, R., MITRA, D. K., TANEJA, V. and GIPHART, M. J. Variants of HLA-DR2/DR51 group haplotypes and susceptibility to tuberculoid leprosy and pulmonary tuberculosis in Asian Indians. Int. J. Lepr. 63 (1995) 241-248.
20. MOSMANN, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65 (1983) 55-63.
21. MUTIS, T., CORNELISSE, Y. E., DATEMA, G., VA N DE N ELSEN, P. J., OTTENHOFF, T. H. M. and DE VRIES, R. R. P. Definition of a human suppressor T-cell epitope. Proc. Natl. Acad. Sci. U.S.A. 91 (1994) 9456-9460.
22. OTTENHOFF, T. H. M., CONVERSE, P. J., GEBRE, N., WONDIMU, A., EHRENBERG, J. P. and KIESSLING, R. The cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur. J. Immunol. 19 (1989) 707-713.
23. OTTENHOFF, T. H. M., HAANEN, J. B. A. G., GELUK, A., MUTIS, T., AB, D. K.., THOLE, J. E. R., VANSCHOOTEN, W. C . A., VAN DEN ELSEN, P. J. and DE VRIES, R. R. P. Regulation of mycobacterial heatshock protein-reactive T cells by HLA class II molecules: lessons from leprosy. Immunol. Rev. 121 (1991) 171-191.
24. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34 (1966) 255-273.
25. SINGH, S. P. N., MEHRA, N. K., DINGLEY, H. B., PANDE, J. N. and VAIDYA, M. C. HLA-linked control of susceptibility to pulmonary tuberculosis and association with HLA-DR types. J. Infect. Dis. 143 (1983) 676-681.
26. TADA, H., SHIHO, O., KUROSHIMA, K., KOYAMA, M. and TSUKAMOTO, K. An improved colorimetric assay for interleukin-2. J. Immunol. Meth. 93 (1986) 157-165.
27. TERASAKI, P. I. and MCCLELLAND, J. D. Microdroplet assay of human serum cytotoxins. Nature 204 (1964) 998-1000.
28. THOLE, J. E., VA N SCHOOTEN, W. C. A., KEULEN, W. J., HERMANS, P. W. M., JANSON, A. A. M., DEVRIES, R. R. P., KOLK, A. H. J. and VA N EMBDEN, J. D. A. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65-kilodalton protein of Mycobacterium bovis BOG. Infect. Immun. 56 (1988) 1633-1640.
29. TOWBIN, H., STAEHELIN, T. and GORDON, J. Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U.S.A. 76 (1979) 4350-4354.
30. VAN EDEN, W., DE VRIES, R. R. P., MEHRA, N. K., VAIDYA, M. C, D'AMARO, J. and VA N ROOD, J. J. HLA segregation of tuberculoid leprosy: confirmaton of DR2 marker. J. Infect. Dis. 141 (1980) 693-701.
31. VA N SCHOOTEN, W. C. A., ELFERINK, D. G., VAN EMBDEN, J. D. A., ANDERSON, D. C. and DE VRIES, R. R. P. DR3 restricted T cells from different HLA-DR3 positive individuals recognize the same peptide aa (2-12) of the mycobacterial 65 kDa heat shock protein. Eur. J. Immunol. 19 (1989) 2075-2079.
32. WATSON, J. D. Prospects for new generation vaccines for leprosy: progress, barriers and future strategics. Int. J. Lepr. 57 (1989) 834-843.
33. YOUNG, D. B., KAUFMANN, S. H. E., HERMANS, P. W. M. and THOLE, J. E. R. Mycobacterial protein antigens: a complication. Mol. Microbiol. 6 (1992) 133-145.
34. YOUNG, D. B., LATHIGRA, R., HENDRIX, R., SWEETSER, D. and YOUNG, R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc. Natl. Acad. Sei. U.S.A. 85 (1988) 4267-4270.
35. YOUNG, R. A., MEHRA, V., SWEETSER, D., BUCHANAN, T., CLARK-CURTISS, J., DAVIS, R. W.and BLOOM, B. R. Genes for major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316 (1985) 450-452.
1. Ph.D.; Histocompatibility and Immunogenetics Department, All India Institute of Medical Sciences, New Delhi 110029, India.
2. Ph.D.; Histocompatibility and Immunogenetics Department, All India Institute of Medical Sciences, New Delhi 110029, India.
3. M.Phil.; Histocompatibility and Immunogenetics Department, All India Institute of Medical Sciences, New Delhi 110029, India.
4. Ph.D.; Histocompatibility and Immunogenetics Department, All India Institute of Medical Sciences, New Delhi 110029, India.
5. Ph.D.; Biotechnology Unit, Chemical Engineering Department, Indian Institute of Technology, Kharagpur, India.
6. M.Sc.; Biotechnology Unit, Chemical Engineering Department, Indian Institute of Technology, Kharagpur, India.
7. Ph.D.; Biotechnology Unit, Chemical Engineering Department, Indian Institute of Technology, Kharagpur, India.
Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India.
Reprint requests to Prof. N. K. Mehra at address above or 91-11-696-7588; FAX: 91-11-686-2663.
Received for publication on 9 March 1995;
Accepted for publication in revised form on 7 July 1995.