- Volume 63 , Number 4
- Page: 539–45
Study on the roles of CD4+ and CD8+ T cells in the expression of host resistance to Mycobacterium leprae infection induced in athymic nude mice
ABSTRACT
The contribution of CD4+ or CD8+ T cells in the host resistance to Mycobacterium leprae was investigated. Athymic BALB/c nude mice were infected subcutaneously with M. leprae into the foot pads 1 or 3 weeks after adoptive transfer with whole, CD4 T cell-depleted, or CD8 T celldepleted lymphocyte fractions prepared f rom spleen cells (SPCs) of euthymic BALB/c mice. SPCs f rom all of the recipient mice showed nearly the same levels of proliferative responses to phytohemagglutinin (PHA) and lipopolysaccharide, except that those of mice transferred with CD4 T celldepleted lymphocytes showed somewhat reduced mitogenic responses to PHA. Significantly potentiated host resistance to the infection was seen, as evidenced by the obviously reduced bacterial growth in all three groups of recipient mice compared to that seen in control mice, that is, 4.7- to 6.4-log unit decreases in the bacterial loads were observed. Levels of the reduction conferred by CD 4 T cell-depleted lymphocytes were comparable to that of CD8 T cell-depleted lymphocytes. This suggests that both CD4 and CD8 T cells are important for the expression of resistance to M. leprae infection.RÉSUMÉ
On a investigué la contribution des cellules T CD4 + et CD8 + dans la résistance de l'hôte au Mycobacterium leprae. Des souris nues athymiques BALB/c ont été infectées par voie sous-cutanéc avec du M. leprae dans le coussinet plantaire des paties une à trois semaines après un transfert de fractions lymphocytaires soit complètes, soit appauvries en cellules T CD4, soit appauvries en cellules T CD8 préparées à partir de cellules spléniqucs (SPC) de souris BALB/c cuthymiques. Les cellules spléniqucs de toutes les souris receveuses montraient presque les mêmes niveaux de réponses prolifëratives à la phytohémagglutinine (PHA) et au lipopolysaccharide, sauf que les cellules spléniqucs des souris ayant reçu des lymphocytes appauvris en cellules T CD4 montraient des réponses mitogéniques à la PHA quelque peu réduites. On a observé une résistance significativement renforcée de l'hôte à l'infection, comme elle a été mise en évidence par la croissance bactérienne visiblement réduite dans les trois groupes de souris receveuses, par comparaison aux souris témoins; c'està-dire qu'on a observé une réduction des charges bactériennes de 4.7 à 6.4 logs. Les niveaux de la réduction provoquée par les lymphocytes appauvris en cellules T CD4 étaient comparables à ceux provoqués par les lymphocytes appauvris en cellules T CD8. Ceci suggère que les cellules T CD4 et CD8 sont loutes deux importantes pour l'expression de la résistance vis-à-vis de l'infection par M. leprae.RESUMEN
Se investigó la contribución de las células TCD4 y TCD8 en la resistencia del huésped a la infección por Mycobacterium leprae. Se inocularon ratones BALB/c desnudos (atímicos) con M. leprae en las almohadillas plantares, una ó 3 semanas después de la transferencia adoptiva de células T totales, de células T depletadas de CD4, o de células T depletadas de CD8 provinientes del bazo de ratones BALB/c eutímicos. Los esplenocitos de los animales que recibieron células T totales o células depletadas de linfocitos TCD8, mostraron respuestas proliferativas comparables cuando se estimularon con fitohemaglutinina (PHA) o lipopolisacárido. Los animales recipientes de células depletadas de linfocitos TCD4 mostraron respuestas proliferativas reducidas. Comparados con los animales control, los animales recipientes de células de los tres grupos mostraron una significativa potenciación de su resistencia a la infección. Esto se dedujo del reducido crecimiento bacteriano en los 3 grupos de animales recipientes (disminuciones en las cargas bacterianas del orden de 4.7 a 6.4 unidades log). Los niveles de reducción de la carga bacteriana conferidos por las células depletadas de linfocitos TCD4 fueron comparables a los conferidos por las células depletadas de linfocitos TCD8. Esto sugiere que que tanto las células CD4 como las células CD8 son importantes en la expresión de resistencia a la infección por M. leprae.Recent studies concerning the immune mechanisms central to the expression of host resistance to Mycobacterium leprae infection have revealed interesting profiles with respect to the T-cell-mediated immune response as follows. First, striking differences were found in the ratio of CD4+ T cells (CD4 T cells) to CD8+ T cells (CD8 T cells) at the poles of the leprosy spectrum (10, 11, 14), i.e., one is the tuberculoid type of leprosy exhibiting strong cellular immunity against M. leprae antigens and showing resistance to the pathogen, and the other is the lepromatous type of leprosy lacking in such an immune response and resistance to pathogens (6). The CD4 and CD8 T-cell populations were found to predominate in tuberculoid and lepromatous lesions, respectively (10, 11, 14). Second, the immunohistologic studies revealed that CD4 T cells present in the center of tuberculoid granuloma are of the T-memory phenotype and located near macrophages, thereby indicating the role of CD4 T cells in mediating accumulation, activation, and maturation of macrophages in the leprosy lesions, leading to restriction and elimination of the organisms (8, 10, 11, 24). On the contrary, in lepromatous lesions, a number of CD8 T cells, expressing the T-suppressor phenotype, were observed to be admixed with macrophages and CD4 T cells, thereby suggesting that CD8 T cells may display their central role in the suppression of the cell-mediated immune response (3, 8, 24). Indeed, CD8 T-cell clones derived from lepromatous leprosy skin biopsies suppressed the proliferative response of HLA-D-matched M. leprae antigen-responsive CD4 T-cell clones (12,18). Third, clear differences in cytokine profiles were found to occur between tuberculoid and lepromatous lesions. Levels of mRNA encoding Type-1 cytokines including interleukin-2 (IL-2) and interferon-gamma (IFN- γ ), produced by Thl cells (1, 15, 18), were higher in tuberculoid lesions than in lepromatous lesions (25), while those encoding Type-2 cytokines, such as IL-4, IL-5, and IL-10, derived from Th2 cells (1, 15, 18), were characteristic of lepromatous lesions (13, 25 ). Thus, Thl cells in CD4 T-cell populations are thought to be involved in cell-mediated immunity to M. leprae infection by producing Type-1 cytokines, especially IFN- γ which potentiates the microbicidal activity of macrophages (17). In contrast, either Th2 cells or Type-2 cytokines, especially IL-4 and IL-10 which also are derived from CD8 T cells possessing suppressor T-cell functions (CD8+ Ts cells) (18), might contribute to the immune unresponsiveness and elevated antibody levels in lepromatous leprosy patients.
These interesting aspects of the immunological profiles of leprosy patients encouraged us to study the role of CD4 and CD8 T cells in detail by using the experimental model of M. leprae infection induced in mice. In this study, we attempted to assess the role of CD4 and CD8 T cells in the expression of host resistance to the pathogens by adoptive transfer of lymphocyte preparations enriched in these T-cell subsets into M. leprae -infected athymic nude mice.
MATERIALS AND METHODS
Mice. Female, 6-week old, BALB/c athymic nude (nu/nu) mice and 8- to 10-week-old, BALB/c euthymic (+/+) mice were purchased from Japan Clea Co., Tokyo, Japan.
Organisms. M. leprae Thai-53 was obtained from Dr. Matsuoka, National Institute for Leprosy Research, Japan.
Special agents. Monoclonal rat anti-L3T4 (CD4) and mouse anti-Lyt-2.2 (CD8) antibodies were obtained from Caltac Laboratories Inc., South San Francisco, California, U.S.A and Cedarlane Laboratories, Ontario, Canada, respectively.
Adoptive lymphocyte transfer. Spleen cells (SPCs) were collected from BALB/c euthymic mice as described previously (23) and adherent cells (mainly macrophages) were eliminated by applying the spleen-cell suspension in RPMI-1640 medium supplemented with 5% fetal bovine serum (FBS) (M. A. Bioproducts, Walkersville, Maryland, U.S.A.) onto a Sephadex G-10 column (30-ml bed volume) followed by a 30-min incubation at 37ºC and subsequent elution with the medium. The resultant lymphocyte fraction was divided into three portions, and 0.8 ml of each of them was treated with either anti-L3T4 antibody (200 µ g/ml); antiLyt-2.2 antibody (200 µ g/ml), or medium alone at 0ºC for 1 hr. After washing with low toxic medium (Cedarlane Cytotoxicity Medium), the cells were suspended in a small volume (ca. 1 ml) of RPMI-1640 medium. Then, 0.2 ml of each of the resultant cell suspensions containing 7 to 8 x 107 cells, i.e., whole lymphocytes, CD4 T-cell-depleted (CD4-depleted) lymphocytes, and CD8 T-cell-depleted (CD8-depleted) lymphocytes, were injected intravenously into BALB/c nude mice.
Blastogenic response of SPCs. SPCs obtained from normal BALB/c euthymic mice and BALB/c athymic nude mice with or without adoptive lymphocyte transfer were measured for the mitogenic response as previously reported (21)- Briefly, 2.5 x 105 of SPCs were cultured in 0.2 ml of 5% FBSRPMI-1640 medium containing either 5 or 10 µ g/ml of phytohemagglutinin (PHA) (Difco Laboratories, Detroit, Michigan, U.S.A) or 50 µ g/ml of lipopolysaccharide (LPS) ( Escherichia coli ; Difco) in triplicate wells of flat-bottom microtiter trays (Corning Glass Works Co., Corning, New York, U.S.A.) at 37ºC in a C02 incubator (5% C02 95% humidified air) for 72 hr. The culture was pulsed with 0.25 µ Ci/well of 3H-thymidine (3H-TdR) (2 Ci/mmol: New England Nuclear Corp., Boston, Massachusetts, U.S.A.) for the final 8 hr. Cells were collected on glass-filter paper and washed with physiological saline using an automatic cell harvester (Mitsumi Kagaku Co., Tokyo, Japan). The radioactivity was counted in a liquid scintillation counter (Tri-Carb Liquid scintillation spectrometer, Packard Instrument Co., Downers Grove, Illinois, U.S.A.).
Experimental infection. M. leprae were harvested from the infected foot pads (FPs) of BALB/c nude mice and a bacterial suspension was prepared as follows (22). The infected FPs were homogenized in Hanks balanced salt solution containing 5% FBS and centrifuged at 200 x g for 5 min. The upper layer was saved and the bacilli were collected by recentrifugation at 1500 x g for 15 min. The bacterial suspension was again centrifuged at 200 x g for 5 min, and the upper layer was used as an inoculum for experimental infection. BALB/c nude mice given or not given adoptive lymphocyte transfer were infected subcutaneously with 1.0 x 106 of M. leprae into the right hind FP. Over a period of 350 days, mice were killed and the number of acid-fast bacilli (AFB) in the FP was enumerated according to the method of Shepard (20).
RESULTS
Reconstitution of T cells in recipient nude mice given lymphocyte transfer from euthymic mice. In order to confirm that our procedures of adoptive lymphocyte transfer gave a sufficient level of reconstitution of recipient nude mice with desired T-cell populations, including whole T cells, CD4-depleted T cells, or CD8-depleted T cells, we measured the proliferative response to PHA and LPS of the SPCs obtained from mice 6 weeks after the T-cell transfer. As shown in Table 1, SPCs obtained from the recipient nude mice transferred with whole or CD8-depleted (enriched in CD4+ CD8- T cells) lymphocytes showed an appreciable degree of mitogenic response to PHA at concentrations of 5 and 10 µ g/ml. On the other hand, SPCs of mice transferred with CD4-depleted lymphocytes showed a lower level of proliferative response to PHA at 10 µ g/ ml, and they displayed no response to PHA at 5 µ g/ml. This finding is not so enigmatic, since CD8+ CD4- T cells differentiated in athymic nude mice are known to show impaired proliferative responses to mitogenic stimuli, such as ConA, despite sufficient levels of IL-2 production and IL-2 receptor expression by them (7). Therefore, it seems that the recipient nude mice were reconstituted with each of the T-cell populations with sufficient cell functions 6 weeks after the adoptive lymphocyte transfer. SPCs of all the test groups of mice showed similar levels of LPS-induced blastogenic responses indicating comparable B-cell function among the three groups.
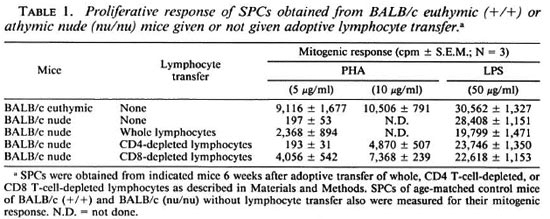
In vivo growth of M. leprae in recipient athymic nude mice given lymphocyte transfer from euthymic mice. Tables 2 and 3 demonstrate the growth of M. leprae inoculated into the FPs of athymic nude mice given or not given adoptive lymphocyte transfer from euthymic mice. In these experiments, mice were infected with M. leprae at 1 (Table 2) or 3 (Table 3) weeks after the transplantation of the indicated lymphocyte preparations enhanced host resistance to M. leprae in the recipient athymic nude mice which were highly susceptible to this infection. Not only the recipients implanted with CD8-depleted lymphocytes but also those transplanted with CD4-depleted lymphocytes showed remarkable levels of resistance to the organisms. It is of interest to note that the CD4-depleted lymphocytes (including CD8+ and double negative T cells) conferred significantly higher (p < 0.05; Student's t test) resistance to the recipient mice as compared to the CD8-depleted lymphocytes (containing CD4+ and double negative T cells).
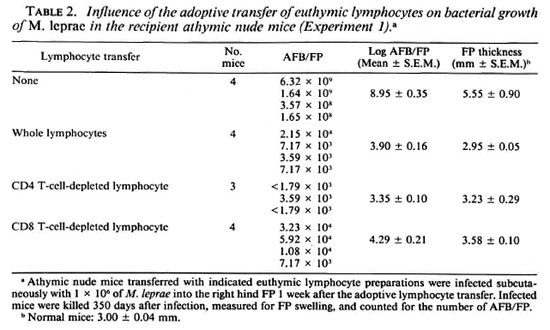
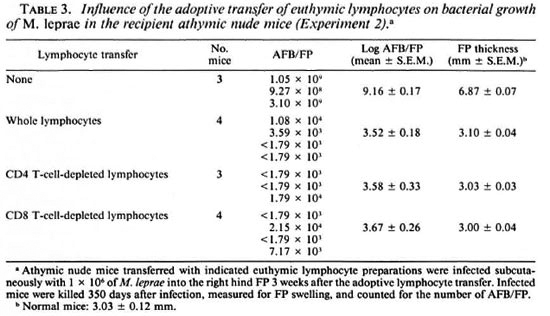
As shown in Table 3, a similar result was obtained when the recipient mice were infected with M. leprae 3 weeks after the lymphocyte transfer. In this case, the bacterial growth was reduced due to the adoptive transfer of whole, CD4-depleted, and CD8depleted lymphocytes in the order of 5.64-, 5.58-, and 5.49-log units, respectively (p < 0.005; Student's t test). Transplantation of CD4-depleted lymphocytes conferred markedly high levels of resistance to M. leprae infection to the recipient mice. However, there was no significant difference in the levels of reduction of the bacterial growth between mice given adoptive transfer of CD4-depleted and CD8-depleted lymphocyte preparations.
DISCUSSION
Previous investigation by Lowe, et al. (9) have demonstrated the ability of adoptively transferred T lymphocytes from M. leprae immunized mice to sublethally irradiated recipient CBA mice to increase host resistance to M. leprae . In relation to this, Shannon, et al. (19) indicated that adoptive transfer of splenic leukocytes from hétérozygote BALB/c (nu/+) mice with or without immunization with M. leprae alone, BCG alone, or a mixture of the two antigens to recipient BALB/c nude mice induced reversal reactions in terms of FP inflammation and swelling, decreased morphological indices of the bacteria, and granuloma formation characterized by lymphocyte infiltration and epithelioid cells. In addition, they showed that the efficacy of donor leukocytes in inducing a response to M. leprae was in the order of cells from mice immunized with M. leprae + BCG, AI. leprae alone, and BCG alone, followed by cells from naive mice. These findings indicate important roles of sensitized T lymphocytes and their immunological reactions in the expression of host resistance to M. leprae .
In this study, we have attempted to evaluate the precise role of CD4 or CD8 T cells in the expression of host resistance to M. leprae by carrying out the adoptive transfer of lymphocyte preparations enriched in the two T-cell subsets into M. leprae -intecied athymic nude mice. As indicated in Tables 2 and 3, both CD4 and CD8 T cells actually participated in the expression of host resistance to the organisms. In particular, the transplantation of euthymic splenic lymphocytes depleted of CD4 T cells (consisting of CD8+ and double negative T cells) conferred somewhat higher resistance than did the lymphocyte preparation depleted of CD8 T cells (consisting of CD4+ and double negative T cells). In CD4 T-cell populations. Th 1 cells are thought to play an important role in the expression of protective immunity to M. leprae infection by mobilizing cellular immunity due to abundant production of Type-1 cytokine, IFN- γ and IL-2 (2, 15, 18, 25).
On the other hand, in CD8 T-cell populations, Tc subsets (Type-1 CD8 T cells) (18) seem to participate in the expression of host resistance, presumably by destroying M. leprae -inkcted macrophages and nonmyeloid cells, which are incapable of mobilizing effective antimicrobial effector mechanisms to the organisms even after lymphokine stimulation, through target-cell lysis, as proposed by Kaufmann, et al. (5, 6) for the case of M. tuberculosis infection. Moreover, the CD8 T cells may up-regulate microbicidal function of macrophages by producing certain kinds of macrophage activating cytokines including IFN- γ , tumor necrosis factor-a, and granulocyte-monocyte colony stimulating factor (GM-CSF) (2, 17). In fact, some CD8 T cells can produce sufficient amounts of IFN- γ after appropriate stimulation with antigen, accessory cells, and IL2, leading to activation of macrophages to exert a substantial level of anti- M. tuberculosis activity (4, 5). In addition, allo-antigen-specific cytolytic CD8 T-cell lines derived from human peripheral blood lymphocytes were found to produce a comparable amount of IFN- γ as compared to Type-1 CD4 T-cell lines (18). In any case, the present finding is consistent with the concept that both CD4 and CD8 T lymphocytes are required for the acquisition and expression of resistance against mycobacterial infections, including tuberculosis (5). However, it may be noted that in M. tuberculosis infection, depletion of either the CD4 or the CD8 T-cell subset resulted in a significant increase in bacterial growth, and CD4 T-cell-depleted mice showed more severe effects (16). This feature is considerably different from our finding as described above, that is, in M. leprae infection, transfer of CD4-depleted lymphocytes caused more reduced bacterial growth than did CD8-depleted lymphocytes (Table 2). Therefore, there may be some difference in the mechanisms of the expression of T-celldependent protective immunity between M. leprae infections and M. tuberculosis infections.
In our recent study, IFN- γ was found to cause a weak elevation of host resistance to M. leprae infection, when given intramuscularly to athymic nude mice at the dose of 104 units/mouse, once weekly from day 91 to 180, causing a 0.35-log unit decrease in the number of AFB in the infected FPs at day 300 after infection, as compared to that of the control mice (manuscript in preparation). The GM-CSF lacked such in vivo activity. Therefore, IFN- γ or GM-CSF alone is not sufficiently efficacious in activating host macrophages to exert antimicrobial activity against M. leprae . IFN- γ may require collaboration with some other lymphokines in order to fully potentiate the microbicidal capacity of macrophages against M. leprae . Our separate experiments also indicated that the combined administration of newly synthesized benzoxazinorifamycin, KRM-1648 (22), with IFN- γ but not with GM-CSF caused appreciably increased therapeutic efficacy against M. leprae in mice. This supports the concept that IFN- γ contributes to the host resistance to M. leprae infection by activating the antimicrobial function of macrophages. Further studies on the roles of various T-cell subsets and their cytokines in the expression of protective immunity against M. leprae infection are under way.
REFERENCES
1. BARNES, P. F., MODLIN, R. L. and ELLNER, J. J. T-cell response and cytokines. In: Tuberculosis. Pathogenesis, Protection, and Control . Bloom, B. R. ed. Washington, DC: ASM Press, 1994, pp. 389-415.
2. BERMUDEZ, L. E. and KAPLAN, G. Recombinant cytokines for controlling mycobacterial infections. Trends Microbiol. 3 (1995)22-27.
3. BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80 ( 1984)5-28.
4. DE LIBERO, G., FLESCH, I. and KAUFMANN, S. H. E. Mycobacteria-reactive Lyt-2+ T cell line. Eur. J. Immunol. 18 (1988)59-66.
5. KAUFMAN, S. H. E. In vitro analysis of the cellular mechanisms involved in immunity to tuberculosis. Rev. Infect. Dis. 11 (1989)S448-S454.
6. KAUFMAN, S. H. E. Cell-mediated immunity. In: Leprosy. 2nd ed. Hastings, R. C , ed. Edinburgh: Churchil Livingstone, 1994, pp. 157-168.
7. KUNG, J.T . Impaired clonal expansion in athymic nude CD8+CD4- T cells. J. Immunol. 140 ( 1988)3727-3735.
8. LONGLEY, J., HAREGEWOIN, A., YEMANEBERHAN, T. WARNDORF-VAN DIEPEN, T., NSIBAMI, J., KNOWLES, D., SMITH, K. A . and GODAL, T. In vivo response to Mycobacterium leprae : antigen presentation, interleukin-2 production, and immune cell phenotypes in naturally occurring leprosy lesions. Int. J. Lepr. 53 (1985)385-394.
9. LOWE, C., BRETT, S. J. and REES, R. J. W. Adoptive cell transfer of resistance to Mycobacterium leprae infections in mice. Clin. Exp. Immunol. 61 (1985)336-342.
10. MODLIN, R. L., HOFMAN, F. M., TAYLOR, C. R. and REA, T. H. In situ characterization of T lymphocyte subsets in leprosy granulomas. Int. J. Lepr. 50 (1982)361-362.
11. MODLIN, R. L., HOFMAN, F. M., TAYLOR, C. R. and REA, T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J. Am. Acad. Dermatol. 8 (1983)182-189.
12. MODLIN, R. L., KATO, H., MEHRA, V., NELSON, E. E., FAX, X.-D., REA, T. H., PATTENGALE, P. K. and BLOOM, B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. (Settu) Nature 322 (1986)459-460.
13. MODLIN, R. L. and NUTMAN, T. R. Type 2 cytokines and negative immune regulation in human infections. Curr. Opin. Immunol. 5 (1993)511-517.
14. MODLIN, R. L. and REA. T. H. Immunopathology of leprosy. In: Leprosy. 2nd ed. Hastings. R. C, ed. Edinburgh: Churchil Livingstone, 1994, pp. 225-234.
15. MOSMANN, T. R., CHEWINSKI, H., BOND, M. W., GIEDLIN, M . A. and COFFMAN, R. L. TWO types of murine helper T cell clones. 1. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136 (1986)2348-2357.
16. MULLER, I., COBBOLD, S. P., WALDMANN, H. and KAUFMANN, S. H. E. Impaired resistance to Mycobacterium tuberculosis infection after selective in-vivo depletion of L3T4+ and Lyt2+ T cells. Infect. Immun. 55 (1987)2037-2041.
17. MURRAY, H. W. Gamma interferon, cytokineinduced macrophage activation, and antimicrobial host defense; in vitro, in animal models, and in humans. Diagn. Microbiol. Infect. Dis. 13 (1990)411-421.
18. SALGAME, P., ABRAMS, J. S., CLAYBERGER, C, GOLDSTEIN, H., CONVIT, J., MODLIN, R. L. and BLOOM, B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 254 (1991)279-282.
19. SHANNON, E. J., CHEHL, S., JOB, C. K. and HASTINGS, R. C. Adoptively transferred reactivity to M. leprae in nude mice infected with M. leprae . Clin. Exp. Immunol. 70 (1987)143-151.
20. SHEPARD, C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 112 (1960)445-454.
21. TOMIOKA, H. and SAITO, H. Characterization of immunosuppressive functions of murine peritoneal macrophages induced with various agents. J. Leukoc. Biol. 51 (1992)24-31.
22. TOMIOKA, H. and SAITO, H. In vivo antilcprosy activity of the newly synthesized benzoxazinorifamycin, KRM-1648. Int. J. Lepr. 61 (1993)255-258.
23. TOMIOKA, H., SAITO, H. and YAMADA, Y. Characteristics of immunosuppressive macrophages induced in spleen cells by Mycobacterium avium complex infections in mice. J. Gen. Microbiol. 136 (1990)965-973.
24. WALLACH, D., FLAGEL, B., BACH, M.-A. and COTTENOT, F. The cellular content of dermal leprous granulomas: an immunohistological approach. Int. J. Lepr. 52 (1984)318-326.
25. YAMAMURA, M., UYEMURA, K., DEANS, R. J., WEINBERG, K., REA, T. H., BLOOM, B. R. and MODLIN, R. L. Defining protective response to pathogens: cytokine profiles in leprosy lesions. Science 254 (1991)277-279.
1. M.D.; Department of Microbiology and Immunology.
2. Ph.D., Department of Microbiology and Immunology.
3. Ph.D., Department of Microbiology and Immunology.
4. Ph.D., Department of Dermatology, Shimane Medical University, Izumo 693, Japan.
5. M.D., Ph.D., National Institute for Leprosy.
Research, Tokyo 189, Japan. Reprint requests to Dr. Tomioka.
Received for publication on 31 May 1995;
Accepted for publication in revised form on 5 September 1995.