- Volume 63 , Number 4
- Page: 546–51
Immune response of armadillos (Dasypus novemcinctus). I. Use of lectins to identify lymphocyte subpopulations and to evaluate cell proliferation
ABSTRACT
Lectins have been used to study populations and discrete differentiation stages of lymphocytes. Likewise, lectins have been of practical importance in promoting mitogenic stimulation of lymphocytes in numerous species. In this research project, we took advantage of these tools in an attempt to identify specific subsets of peripheral blood lymphocytes obtained f rom healthy nine-banded armadillos (Dasypus novemcinctus). The same cell source served to evaluate mitogenic stimulation. Twelve FITC-labeled lectins were used; 5 (ConA, LcH, RCA, WGA and UEA) reacted with almost 100% of the lymphocytes and 7 (PNA, DBA, SBA, PCA, PHA-L, PWM and VVA) recognized variable percentages (< 100% of these cells). This latter group of lectins may be useful in the identification of armadillo lymphocyte subsets, or may correlate with discrete stages of differentiation of these cells. The same lectins served to evaluate mitogenic stimulation in an aliquot of the same peripheral blood mononuclear cells. Of the 12 lectins studied, 5 (ConA, PHA-L, PWM, DBA and SBA) had the capacity to induce mitogenic stimulation in the whole mixture of mononuclear cells, giving rise to variable degrees in the corresponding mitogenic index obtained for each of the 5 lectins. Those lectins that gave an indication of selective identification of lymphocytes, that is, the percentages at or below 75%, may prove useful in the evaluation of the immune response of healthy armadillos as well as the evolution of progression stages of lepromatous leprosy in armadillos inoculated with the same strain of Mycobacterium leprae that affects humans.RÉSUMÉ
Les lectines ont été utilisées pour étudier des populations de lymphocytes et leurs stades de differentiation. De même, les lectines ont été importantes pour promouvoir la stimulation mitogénique des lymphocytes dans de nombreuses espèces. Dans ce projet de recherche, nous avons profité de ces outils pour essayer d'identifier des sous-groupes spécifiques de lymphocytes du sang périphérique provenant de tatous à neuf bandes en bonne santé (Dasypus novemeinctus). La même source de cellules a servi à évaluer la stimulation mitogénique. Douze lectines marquées au FITC ont été utilisées; cinq (ConA, LcH, RCA, WGA et UEA) réagissaient avec près de 100% des lymphocytes et sept (PNA, DBA, SBA, PCA, PHA-L, PWM et VVA) en reconnaissaient des pourcentages variables (< 100% de ces celulles). Ce dernier groupe de lectines pourrait être utile dans l'identification de sous-populations de lymphocytes de tatous, ou pourrait être associé avec des stades de differentiation de ces cellules. Les mêmes lectines ont servi à évaluer la stimulation mitogénique dans un aliquot des mêmes cellules mononucléaires du sang périphérique. Parmi les douze lectines étudiées, cinq (ConA, PHA-L, PWM, DBA et SBA) étaient capables de provoquer la stimulation mitogénique dans le mélange complet de cellules mononucléaires, donnant lieu à des degrés variables de l'indice mitogénique correspondant obtenu pour chacune des cinq lectines. Les lectines qui donnaient une indication de l'identification sélective de lymphocytes, c'est-à-dire un pourcentage de ou inférieur à 75%, pourraient s'avérer utiles dans l'évaluation de la réponse immunitaire des tatous en bonne santé ainsi que de l'évolution des stades de progression de la lèpre lépromatcuse chez des tatous auxquels on a inoculé la même souche de Mycobacterium leprae que celle qui affect l'homme.RESUMEN
Se han usado lectinas para estudiar las poblaciones de linfocitos y sus discretos estados de diferenciación. Las lectinas han resultado ser de importancia práctica al promover la estimulación mitogénica de linfocitos de diversas especies. En este estudio aprovechamos esta herramienta para intentar identificar subpoblaciones específicas de linfocitos de la sangre periférica de armadillos sanos de nueve bandas (Dasypus novemcinctus). La misma fuente celular se usó para evaluar la estimulación mitogénica. Se usaron 12 lectinas acopladas a fluoresceína; cinco de ellas (ConA, LcH, RCA, WGA y UEA) reaccionaron con casi el 100% de los linfocitos, y 7 (PNA, DBA, SBA, PCA, PHA-L, PMW y WA) reconocieron porcentajes variables (<100%) de estas células. Este último grupo de lectinas podráia de ser de valor para identificar subpoblaciones de linfocitos de armadillo o para identificar discretos estados de diferenciación de estas células. Las lectinas también se usaron para evaluar su capacidad mitogénica sobre la población de leucocitos mononucleates de sangre periférica. Délas 12 lectinas estudiadas, 5 (ConA, PHA-L, PWM, DBA y SBA), indujeron la mitosis de las células mononucleares, aunque en diferentes grados y con diferentes índices de estimulación. Aquellas lectinas cuyo comportamiento podría sugerir una identificación selectiva de linfocitos, esto es, que mostraron porcentajes de estimulación iguales o menores al 75%, podrían ser útiles para evaluar la respuesta inmunológica de los armadillos sanos y para seguir el progreso de la enfermedad en los armadillos infectados con Mycobacterium leprae.The nine-banded armadillo (Dasypus novemcinctus) has become the main source for growing Mycobacterium leprae although very little is known about its immune response. However, the knowledge of the mechanisms involved in protection could be relevant to understanding why armadillos develop an infection quite similar to the lepromatous leprosy seen in humans.
There is a scarcity of publications with reference to elements of the immune response in armadillos (3, 17). Some reports give fragmentary information on the humoral branch only. As far as we know, there are no reports on the identification of lymphocyte surface markers, indispensable for a breakdown of the different cell types or subpopulations making up the cellular branch of immunity, which participate in the area of our investigative concern (1, 3, 12, 17).
In this paper we report on the use of lectins to identify lymphocyte subpopulations and to evaluate cell proliferation in the armadillo. Lectins have proven to be useful in the identification of certain moieties of the lymphocyte population (14), and even of value in pinpointing distinct stages of differentiation (7). Lectins have the additional property of inducing polyclonal activation of lymphocytes (7, 8) and the release of cytokines. There exist only a limited number of references on the response of armadillo lymphocytes (9, l8). For this reason, a careful revision of data pertaining to the stain pattern of peripheral lymphocytes from armadillos in response to lectin stimulation constitutes a distinctive contribution of this presentation. The use of 12 different lectins was involved in the study of the proliferation of the peripheral lymphocytes with the intention of establishing the features related to the functional subtleties that may help define subpopulations of armadillo lymphocytes.
MATERIALS AND METHODS
Armadillos. Nine-banded armadillos ( Dasypus novemcinctus ) were captured in the highlands of the state of Michoacan, Mexico, and were kept in captivity as previously described (10). After 1-3 months of adaptation, 20 ml of blood was withdrawn from each animal by cardiac puncture (12). Blood was mixed with an equivalent volume of Alsever's solution to avoid coagulation.
Purification of lymphocytes from peripheral blood. The mononuclear cells were purified from peripheral blood using Histopaque 1077 (Sigma Chemical Co., St. Louis, Missouri, U.S.A) as was described by Boyum (2). After purification, half of the cells were resuspended in complete medium for culture and the other half were resuspended in PBA [phosphate buffered saline (PBS) to which was added 2% bovine serum albumin (BSA) and 0.01% NaN3] for staining.
Medium. RPMI-1640 (Gibco, Grand Island, New York, U.S.A.) was supplemented with nonessential amino acids (Gibco), 5 x 10-5 M 2-mercaptoethanol (Gibco), 1 mM sodium pyruvate (Sigma), 2 mM glutamine (Sigma) and 5% (v/v) fetal calf serum (Gibco). Medium with all the supplements isreferred to as complete medium.
Lymphocyte staining. Armadillo mononuclear cells (1 x 106 cells/tube) in PBA were stained with different concentrations of FITC-labeled lectins. Cell suspensions were incubated for 30 min at 4ºC. After incubation, the cells were washed three times with PBA, and the fluorescent cells were scored by direct observation under epifluorescence microscopy (Carl Zeiss, Germany).
Lectins. All FITC-labeled (for staining) or unlabeled (for tissue culture) lectins used in this work were obtained from Sigma, and they were as follows: Arachis hypogaea (peanut, PNA); Canavalia eisiformis (Concanavalin A from jack bean, ConA); Dolichus biflorus (horse gram, DBA); Glycine max (soybean, SBA); Lens culinaris (lentil, LcH); Phaseolus coccineus (scarlet runner bean, PCA); Phaseolus vulgaris (red kidney bean, PHA-L); Phytolacca americana (pokeweed, PWM); Ricinus communis (castor bean, RCA); Triticum vulgaris (wheat germ, WGA); Ulex europaeus I (gorse or furze, UEA I) and Vicia villosa (hairy vetch, VVA).
Lymphocyte proliferation assays. Armadillo lymphocytes in complete medium were placed into 96-well flat-bottom plates at 1 x 105 cells/well. Lectins diluted in complete medium were added at different concentrations to each well in a total volume of 200 µ l. Plates were incubated at 37ºC, 95% humidity and 5% CO2 for 48 hr. Proliferation was evaluated by 3H-thymidine incorporation (Amersham International, Amersham, Buckinghamshire, U.K.) using 0.5 µ Ci/well during an 8-hr pulse, 3H-thymidine incorporated into the cells was quantified in a Beckman Scintillation counter.
RESULTS
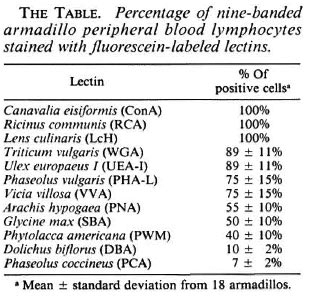
A panel of 12 lectins were used to identify lymphocyte subpopulations in nine-banded armadillos; the results of the staining are shown in The Table. As can be seen, ConA, LcH, RCA, WGA and UEA-I stained almost 100% of the lymphocytes. PHA-L and VVA recognized 75% of the mononuclear cells; PNA, SBA and PWM stained around 50% of the cells; and finally, DBA and PCA reacted with about 10% of the lymphocytes. In this work, all lectins staining less than 75% of the cells were considered good candidates for recognizing subsets of armadillo lymphocytes.
The same lectins also were tested for their capacity to induce proliferation of armadillo lymphocytes. From all of the lectins tested only ConA, DBA, SBA, PHA-L and PWM were able to induce proliferation. As can be seen in The Figure, ConA, PHA-L and PWM induced high levels of 3H-thy midine incorporation. DBA and SBA induced poor proliferation (The Figure); however, the effect was titratable. All of the other lectins used in this work were unable to induce proliferation at any of the concentrations tested.
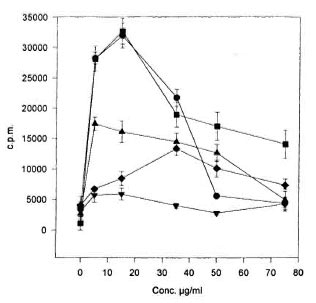
The Figure. Proliferation of armadillo peripheralblood lymphocytes induced by lectins. Armadillo lym-phocytes were stimulated with different concentrationsof the following lectins: ConA  , PHA-L
, PHA-L  , PWM
, PWM  , DBA
, DBA  and SBA
and SBA  . After a 40-hr incubation,plates were pulsed for 8 hr with 3H-thymidine and thenharvested. Every well was done in quadruplicate. Eachpoint represents the mean ± standard deviation fromsix different healthy armadillos.
. After a 40-hr incubation,plates were pulsed for 8 hr with 3H-thymidine and thenharvested. Every well was done in quadruplicate. Eachpoint represents the mean ± standard deviation fromsix different healthy armadillos.
The optimal concentration for proliferation with ConA, PHA-L PWM and DBA was in the range between 5 to 15 µ g/ml as reported when human or mouse lymphocytes are used. In contrast, 35 µ g/ml of SBA were needed to obtain the highest proliferation with this lectin (The Figure).
DISCUSSION
Information on specific surface markers for identification of subpopulations of peripheral lymphocytes from nine-banded armadillos is very limited, and insufficient for a clear separation into B or T lymphocytes. Some evidence supports the possibility of the identification of B cells (12, 17).
In the literature one finds reports of proliferative responses of armadillo lymphocytes to lectins (9, 18). In those same reports the authors provide proof of mitôgenic stimulation by PHA-L and PWM lectins, both exhibiting good stimulation indexes.
Lectins have proven to be adequate tools for the identification of surface markers in lymphocytes and to promote proliferative responses in those cells from various species. This has encouraged our investigation of the effects of a panel of 12 lectins upon peripheral lymphocytes obtained from the nine-banded armadillo. The staining experiments showed that ConA, LcH, RCA, WGA, UEA-I, PHA-L and VVA lectins recognize high percentages of armadillo lymphocytes. The high percentage of staining means that these lectins will not be useful in identifying specific lymphocyte subpopulations. Nevertheless, they can be helpful in double-staining techniques to confirm identification of the type of cells, or in combination with recognition of lymphocytes identified by the presence of a surface immunoglobulin. Ongoing work in our laboratory is indicating that double staining (with anti-armadillo IgM and lectins) may be useful to identify armadillo lymphocyte subpopulations. However, it is too early to clearly relate phenotype with specific functions.
PHA-L and PWM arc the lectins often selected for characterization and differentiation of human and murine T and B lymphocytes. PHA-L lectin recognizes 75% of T lymphocytes in both species; on the other hand, the PWM lectin identifies 30% to 40% of the lymphocytes, these cell comprise the corresponding B-cell subpopulations (4, 5, 19). In the peripheral blood lymphocytes of the armadillo PHA-L and PWM lectins stain 75% and 40% of the subpopulations, respectively, highly indicative that here, too, there is selective differentiation of the mononuclear cells in this species.
Seventy-five percent of the armadillo lymphocytes were stained by the VVA lectin. In humans, this lectin recognizes 15% of the human peripheral blood lymphocytes, cells with so-called contrasupressor activity (6). Other reports mention that this lectin, in mice, binds preferentially to T cells in the intestinal distribution of Peyer's patches. Furthermore, it has been claimed that the cells identified by VVA lectin secrete interleukin 5 (15). The percentage found in the current study is higher than the values reported in humans, for which reason it is not possible, as yet, to speculate about its possible function in armadillos.
Fifty percent of the armadillo peripheral lymphocytes were stained by PNA as well as SBA. In human and in murine peripheral lymphocytes the PNA lectin stains 40% of these cells, while 80% of thymocytes take up the stain in both of these species (11, 13). In humans, SBA lectin recognizes, unselectively, T and B lymphocytes, but in mice it has been reported that this lectin may bind to the interleukin-2 receptor or other activation markers on T cells (16).
Low percentages of armadillo peripheral blood lymphocytes are stained by DBA and PCA lectins. No reference seems to exist on DBA lectin differentiation of human or murine lymphocyte subsets. However, DBA lectin has the property to differentiate human Al from A2 blood groups (13).
One interesting characteristic of the DBA lectin brought out in the course of the present study is its ability to induce lymphocyte proliferation, an activity that had not been previously reported. The proliferation that was observed, while discrete, is significant, especially if one considers that only 7% of the total lymphocyte population is stained by this lectin. In contrast, a relatively high percentage, 33%, has been reported in the corresponding cell population of mice (13).
The mitogenic stimulation of armadillo peripheral blood lymphocytes with ConA, PHA-L, PWM and DBA lectins was achieved by 5 µ g of any of these four lectins; the first three of these lectins are routinely used for promoting lymphocyte proliferation in humans and in mice (4, 19). In the case of SB A, a higher concentration was required, and the stimulation index was lower compared to the other lectins (ConA, PHA-L and PWM). Enhancement of proliferation may be attained by treatment of the lymphocytes with neuraminidase prior to the culture of the lectin lymphocyte mixture.
The proliferative response of M. leprae infected armadillos is being evaluated in our laboratory, and some preliminary results have shown a decrease in the response to ConA correlating with the progress of the infection.
The results of this investigation have not yielded sufficient information for detection of specific surface markers of peripheral blood T and B lymphocytes obtained from the nine-banded armadillo. However, valuable information has been obtained that is expected to be applicable for identification of cell functions when specific subsets of lymphocytes can be confidently identified. The information regarding selective staining of lymphocyte subsets is of major importance in view of the fact that the armadillo is the only animal species that can serve as an experimental model for the study of the inoculation and spread with M. leprae .
Acknowledgment. This work was supported by grants from Consejo Nacional de Ciencia y Tecnología, México D.F., México, and Dirección de Estudios de Postgrado e Investigación, I.P.N., México. The authors hold fellowships from COFAA, EDD and/or SNI, Mexico. We thank Dr. Cesar Calva Pellicer for his critical reading of this manuscript.
REFERENCES
1. BALIÑA, L. M., CARDAMA, J. E., GAHI, J. C, VALDEZ, R. P. and BIANCHI, O. Research on armadillos in Argentina. In: The Armadillo as an Experimental Model in Biomedical Research . Washington, DC: Pan American Health Organization, 1978, pp. 103-106. Sci. Publ. 366.
2. BOYUM, A. Isolation of lymphocytes, granulocytes and macrophages. Scan. J. Immunol. 5(1976)9-15.
3. ESCOBAR-GUTIERREZ, A. and AMEZCUA, M. E. El armadillo: un nuevo animal de experimentación para el estudio de las zoonosis. Ciencia Veterinaria. Edit. Ricardo Moreno Chan. UNAM, México. 3(1981)200-224.
4. HERNÁNDEZ, D. E. and LEAVITT, R. D. Mitogenic and mitogcnically defective phytohcmagglutinin isolcetins stimulate T cell growth factor (Intcrlcukin 2) production and response in fresh and cultured human T lymphocytes. Cell. Immunol. 86(1984)101-108.
5. KJMURA, A. K. The fine specificity of lectins applied to the study of lymphocyte membrane structure and immunological reactivity. EOS 2(1982)117-124.
6. LEHNER, T., AVERY, J. and JONES, T. Separation and characterization of a subset of human T8 + cells which function as antigen-presenting and contrasuppressor cells. Immunology 54(1985)713-723.
7. LIS, H. and SHARON, N. Lectins as molecules and as tools. Ann. Rev. Biochem. 55(1986)35-67.
8. PEACOK, J. S., COLSKY, A. S. and PINTO, V. B. Lectins and antibodies as tools for studying cellular interactions. J. Immunol. Methods 126(1990)147-157.
9. PURTILO, G. P. and STORRS, E. E. Impact of cool temperatures on transformation of human and armadillo lymphocytes ( Dasypus novemeinctus Linn ) as related to leprosy. Nature 248(1974)450-452.
10. QUESADA-PASCUAL, F., ROJAS-ESPINOSA, O., SANTOS-ARGUMEDO, L. and ESTRADA-PARRA, S. A Mexican armadillo (Dasypus novemeinctus) colony for leprosy research. Int. J. Lepr. 55(1987)716-718.
11. ROSE, M. L. Binding of peanut lectin to lymphocytes: a marker of immaturity. Immunol. Today 3 (1982)25-26.
12. SANTOS-ARGUMEDO, L., GUERRA-INFANTE, F., QUESADA-PASCUAL, F. and ESTRADA-PARRA, S. Identification and purification of armadillo (Dasypus novemeinctus) immunoglobulins: preparation of specific antisera to evaluate the immune response in these animals. Int. J Lepr. 63(1995)56-61.
13. SHARON, N. Lectin receptors as lymphocyte surface markers. Adv. Immunol. 34(1983)213-298.
14. SHARON, N. and Lis, H. Lectins . London: Chapman and Hall Ltd., 1989.
15. SHOENBECK, S., HAMMEN, M. J. and KAGNOFF, M. F. Vicia villosa agglutinin separates freshly isolated Peyer's patch T cells into interleukin 5- or interleukin 2- producing subsets. J. Exp. Med. 169(1989)1491-1496.
16. SOWALSKY, R. A. and Fox, B. S. Pattern of lectin binding to murine T lymphocytes. Immunology 75(1992)92-98.
17. STORRS, E. E., W ALSH, G. P. and B URCHFIELD, H. F. Development of leprosy in another species of armadillo Dasypus hybridus; genetic and immunologic implications. J. Trop. Med. 78(1975)216-218.
18. ULRICH, M., C ONVIT, J., C ENTENO, M. and RAPETTI, M. Immunological characteristics of the Immune Response of Armadillos 551 armadillo Dasypus sabanicola. Clin. Exp. Immunol. 25(1976)170-176.
19. YACHNIN, S. and S VENSOSN, R. The immunological and physicochemical properties of mitogenic proteins derived from Phaseolus vulgaris . Immunology 22(1972)871-890.
1. Ph.D., Cell Biology Department, Centro de Investagacion y Estudios Avanzados, IPN, Apartado Postal 14-740, Mexico, DF 07000, Mexico.
2. M.Sc.; Immunology Department, Escuela Nacional de Ciencias Biológicas, IPN, Prol. Carpió y Plan de Ayala, Mexico, DF 11340, Mexico.
3. B.Sc; Immunology Department, Escuela Nacional de Ciencias Biológicas, IPN, Prol. Carpió y Plan de Ayala, Mexico, DF 11340, Mexico.
4. M.Sc;Immunology Department, Escuela Nacional de Ciencias Biológicas, IPN, Prol. Carpió y Plan de Ayala, Mexico, DF 11340, Mexico.
5. Ph.D., Immunology Department, Escuela Nacional de Ciencias Biológicas, IPN, Prol. Carpió y Plan de Ayala, Mexico, DF 11340, Mexico.
Reprint requests to Dr. Santos-Argumedo at address above or FAX = 525-747-7081, email = santos@cell. cinvestav.mx.
Received for publication on 7 June 1995;
Accepted for publication in revised form on 28 September 1995.