- Volume 63 , Number 4
- Page: 573–4
Differences in M. leprae-induced nerve damage in swiss white and C57BL/6 mice
To the Editor:
Based on our earlier observations that Schwann cells (2) and macrophages(1, 4) of Swiss white and C57BL/6 mice respond differently to Mycobacterium leprae infection, the present study was undertaken to determine if this difference was reflected in the pattern of nerve damage induced by M. leprae in these two strains.
The mice were inoculated with 104 M. leprae in each hind foot pad. At regular time intervals, the mice were anesthetized with pentobarbitone and the sciatic nerve biopsies were obtained. The biopsies were fixed in 2.5% glutaraldehyde, post-fixed in osmium tetroxide, and embedded in araldite. Semithin sections l- µ m thick stained with toluidine blue were used for light microscopy, and subsequent ultrathin sections stained with uranyl acetate and lead citrate were observed under the electron microscope. After the nerve biopsies were collected the mice were killed and the foot pad harvests done according to the method of Rees (5).
M. leprae growth in the mouse foot pad was comparable in the two strains up to the 20th post-inoculation month.
The pathology observed in the sciatic nerves of M. leprae -inoculated Swiss white mice was similar to the early changes seen in leprosy patients (6, 7): at 6-8 months postinoculation there was an involvement of predominantly unmyelinated fibers which progressed to extensive demyelination by the 20th post-inoculation month (Fig. 1). In the C57BL/6 strain, however, while the unmyelinated fiber involvement at 8th month post-inoculation was comparable to the Swiss white mice, it did not progress further to demyelination even though the acid-fast bacilli count at the 20th month was similar in both strains (Fig. 2).
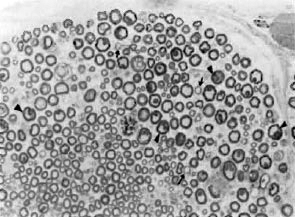
Fig. 1. Part of the sciatic nerve from a Swiss whitemouse inoculated in the foot pad with M. leprae 20 months prior to biopsy. Increased interfiber space seen, suggestive of loss of myelinated fibers. Also present are small thinly myelinated fibers (arrows) and several large myelinated fibers with highly irregular myelin (arrowheads), indicating remyelination and atrophic changes respectively (araldite-embedded tissue, I - µ m thick section stained with toluidine blue x 200).
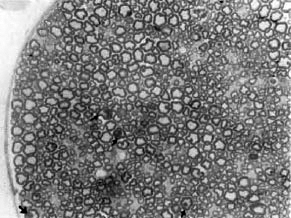
Fig. 2. Part of the sciatic nerve from a C57B/6 mouse inoculated in the foot pad with M. leprae 20 months prior to biopsy. Note that no significant fiberloss or regenerating units are seen in this nerve. However, there are a few large myelinated fibers with irregular myelin (arrows) suggestive of atrophy (aralditeembedded tissue, 1- µ m thick section stained with toluidine blue x 200).
These differences in nerve damage patterns in the two strains may be due to the differential Schwann cell functions of providing immunological sensitization (3) and the differential expression of NGF receptor and cell-adhesion molecules (unpublished observations).
- Tannaz J. Birdi, Ph.D.
Vanaja P. Shetty, Ph.D.
Noshir H. Antia, F.R.C.S., F.A.C.S.(Hon.)
The Foundation for Medical Research
84-A R.G. Thadani Marg
Worli
Bombay 400 018, India.
Acknowledgment. This work was supported by grant no. 030074/89 from The Wellcome Trust, U.K.
REFERENCES
1. BIRDI, T.J., SALOAME, F.R. and ANTIA, N.H. The role of macrophages in leprosy as studied by protein synthesis of macrophages from resistant and susceptible hosts-a mouse and human study. Lepr. India 52(1979)23-42.
2. MEHTA, R., BIRDI, T.J. and ANTIA, N.H. Etfect of St. leprae-infected Schwann cells and their supernatant on lymphocyte neuroglia interaction. J. Neuroimmunol. 22(1989)149-155.
3. MISTRY, N.F., SHETTY, V., SHETTY, V.P. and ANTIA, N.H. Ability and functional implications of sensitization of lymphoid cells in vitro by mycobacterial infected Schwann cells. In: Principles of Design and Functioning in the Nervous System. Singh, N., ed. New Delhi: Wiley Eastern Ltd., 1992, pp. 437-450.
4. NAIR, I., VARADKAR, D. and MAHADEVAN, P.R. Viability of M. leprae inside macrophages from different strains of mice and possible genetic control. Int. J. Lepr. 58(1989)548-553.
5. REES, R.J.W. Limited multiplication of AFB in foot pads of mice inoculated with M. leprae . Br. Exp. Pathol. 45(1964)207-218.
6. SHETTY, V.F., ANTIA, N.H. and JACOBS, J.M . The pathology of early leprous neuropathy. J. Neurol. Sci. 88(1988)115-131.
7. SHETTY, V.P., VIDYASAOAR, F.B. and ANTIA, N.H. Study of evaluation of nerve damage in leprosy. Part III. Sciatic nerve lesions in mice inoculated with M. leprae with nerve conduction velocity correlates. Indian J. Lepr. 52(1980)26-47.