- Volume 63 , Number 3
- Page: 382–91
Relapses during long-term follow up with drug-susceptible M. leprae among multibacillary leprosy patients treated with multidrug therapy regimens; case reports
ABSTRACT
A controlled clinical trial in highly bacilliferous multibacillary leprosy patients was initiated in 1977. We report here two cases of relapse during long-term follow up of patients 15 years after the start of treatment. The patients reported here were treated with rifampin, isoniazid, clofazimine and dapsone for the first 3 months followed by clofazimine and dapsone until 84 months in one case; the other case received the same treatment but had received dapsone alone for 60-84 months. The relapses occurred 6 ½ and 7 ½ years after therapy was discontinued.RÉSUMÉ
On a commencé en 1977 un essai clinique contrôlé chez des patients lépreux multibacillaires hautement bacillifercs. Nous rapportons ici deux cas de rechute durant le suivi à long terme des patients 15 ans après le début du traitement. Les patients rapportés ici avaient été traités par rifampicine, isoniazidc, clofazimine et dapsonc pour les trois premiers mois, puis par clofazimine et dapsone jusqu'au quatre-vingt-quatrième mois dans un cas; l'autre cas avait reçu le même traitement, mais avait reçu la dapsonc seule du soixantième au quatre-vingt-quatrième mois. Les rechutes sont survenues 6½ ans et 7 ½ ans après l'arrêt du traitement.RESUMEN
En 1977 se inició un estudio clínico controlado en pacientes con lepra lepromatosa altamente bacilíferos, con objeto de establecer la frecuencia de recaída de la enfermedad. Aquí se reporta el caso de 2 pacientes que mostraron recaída después de 15 años de haber iniciado el tratamiento antileproso. Uno de los pacientes fue tratado con rifampina, isoniazida, clofazimina y dapsona durante los primeros tres meses y después con clofazimina y dapsona hasta los 84 meses; el otro caso recibió el mismo tratamiento pero recibió dapsona sola durante los meses 60-84. Las recaídas ocurrieron, respectivamente, a los 6.5 y 7.5 años de haber suspendido el tratamiento.Treatment of leprosy was revolutionized with the advent of multidrug therapy (MDT) regimens. The advantages expected were shortening the duration of treatment and the duration of surveillance. The report of a case of relapse 52 months after stopping MDT (2) raises the question whether short-term follow up (5 years), usually recommended after stopping MDT, is adequate. We report here two cases of relapse in the late stages of follow up after stopping treatment.
Case 1. In October 1978, Mr. R., a male aged 45 years, was diagnosed as having lepromatous leprosy which was confirmed by histopathology. Bacteriological examination of the skin from six sites was highly positive for acid-fast bacilli (AFB) (BI 5.33 with MI 0.25%). He was allocated to a multidrug regimen which included rifampin (600 mg), clofazimine (100 mg), dapsone (100 mg) and isonazid (300 mg) daily for the first 3 months followed by clofazimine (100 mg) and dapsone (100 mg) daily up to 60 months, followed by clofazimine (50 mg) plus dapsone (100 mg). At the end of 84 months his BI was 1.00, and he was allocated to a placebo group. The course of the disease was uneventful and his skin smears became negative by 114 months and remained so until 156 months except for a low positive (BI 0.17, three granular bacilli in one site) at 120 months. Clinically, his lesions were inactive and showed marked improvement.
During routine clinical examinations at 162 months, it was found that he had developed nodular skin lesions over the elbows, knees and back of the foot. Skin smears for AFB showed a BI of 2.75 and a MI of 0.19%. Skin biopsy was done for histopathological examination and mouse foot pad experiment (MFE). Histopathological classification was borderline lepromatous leprosy. MFE was done to assess the viability and susceptibility of the bacilli to drugs. The bacillary count in the inoculum was 104/0.03 ml and MI 4%. The drug susceptibility test with dapsone, rifampin and clofazimine showed that the M. leprae strain isolated was susceptible to all three drugs. The patient was treated with rifampin, clofazimine and dapsone [National Leprosy Program (NLEP) regimen], and he responded well.
Case 2. In December 1977, Mr. K., a male aged 45 years, was diagnosed clinically as having leprosy of the lepromatous type which was confirmed by histopathology. Bacteriological examination of the skin was highly positive (BI 3.66). He was allocated to a drug regimen similar to Case 1. At month 60 he was prescribed dapsone alone for 2 years and a placebo thereafter. He showed marked clinical and bacteriological improvement. He did not experience lepra reaction except for a mild attack of neuritis. At 114 months, his BI was found to be negative and remained so until 168 months.
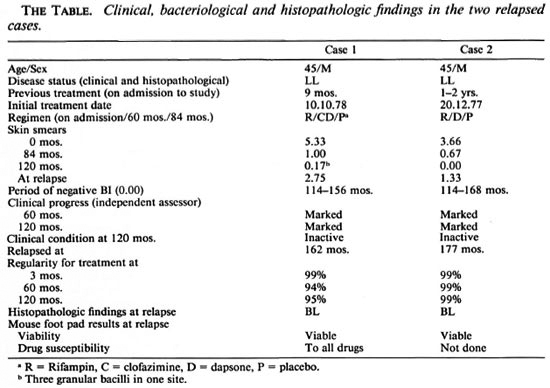
During routine clinical examination at 177 months hypopigmented hypoesthetic patches were noticed on the trunk and extremities but the patient was not aware of their presence. A bacteriological examination of his skin showed a BI of 1.33 and a histopathological classification of borderline lepromatous leprosy was made. The bacillary count in the inoculum was 104 AFB/0.03 ml. There was a tenfold increase in AFB, showing that the organisms had multiplied in the mouse foot pad. Drug sensitivity testing could not be done, but the patient responded to retreatment with the NLEP regimen.
DISCUSSION
Although the treatment of leprosy has been revolutionalized by the introduction of MDT (6) the end point of treatment has not been established. Both of our cases reported here were participants in the clinical trial that was initiated in 1977 to evaluate the efficacy of MDT in lepromatous and borderline lepromatous leprosy cases with Bis of > 2.5. Patients were randomly allocated to either a four-drug regimen of rifampin plus clofazimine plus dapsone plus isoniazid (all drugs daily under supervision for the first 3 months) followed by clofazimine plus dapsone daily for 57 months, or a two-drug regimen of clofazimine plus dapsone daily for 5 years.
At the end of 5 years it was found that some of the patients still had positive Bis and so it was considered unethical to stop treatment. Since most of the patients had granular bacilli and a BI around 1 + , it was decided to stop clofazimine in half the pa tients. These patients were randomly allo cated to either a regimen with clofazimine plus dapsone or dapsone alone for a period of 2 years. Encouraged by the steady fall in the Bis and excellent clinical improvement, a decision was taken to stop treatment in half of the patients and continue dapsone in the other half. Both of the relapsed pa tients reported here belonged to the regimen receiving rifampin daily for the first 3 months and a placebo at 84 months.
During the intensive phase of first 3 months (daily supervised administration of drugs) both of them had received 99% of their treatment; subsequently it was 99% in Case 1 and 94% in Case 2 until the end of chemotherapy (84 months). They relapsed 6½ years and lxh years after stopping drugs and after remaining negative for 3½ and 4½ years, respectively. Mouse foot pad exper iments showed that Mycobacterium leprae isolated from Case 1 were susceptible to all three drugs (rifampin, clofazimine and dapsone). A susceptibility test could not be done for Case 2 but he also responded well to the NLEP regimen, indicating that he probably was harboring susceptible organisms.
The duration and rhythm of rifampin administration still remains controversial. It was in 1977, i.e., 4 years ahead of the introduction of the WHO regimen, that the reported regimens were initiated. WHO believed that there is no evidence to indicate that the monthly treatment with rifampin is inferior to daily treatment with that drug. This view has been refuted (1) by the evidence that viable M. leprae decline at a rate of at least 0.249/day during daily rifampin but not more than 0.053/day during treatment with monthly rifampin.
A recent animal experiment report strengthens the above statement (4) wherein nude mice were treated with five types of regimens: a) continuous rifampin, b) continuous dapsone, c) continuous clofazimine, d) continuous all three drugs, e) rifampin monthly. All treatments were given for 1 year. In animals given monthly rifampin, the passaged bacilli grew in all the animals inoculated, but there was no growth in those mice receiving a continuous dose of the drugs. Based on this report, we conclude that our patients also have received adequate treatment, i.e., 3 months of continuous rifampin that covers three generation times of the bacilli, along with continuous dapsone and clofazimine. Even though they received rifampin for only 3 months it must be remembered that they had received clofazimine plus dapsone continuously for 5 years.
The results of the THELEP trials(5) demonstrate that persisting M. leprae were detected in approximately 9% of all patients without relation to regimen or duration of treatment. However, the follow up in that trial was confined to 3 years. The present report emphasizes the value of long-term follow up (more than 5 years) in a disease with a very long incubation period.
This report highlights an important problem in the management of leprosy. Late relapses now seem to be a feature of the disease as shown in this report. The cause of these relapses may be either due to persisters or due to re-infection. In the absence of any method to prove re-infection, it is reasonable to assume that the relapses are caused by persisters. In this context it is interesting to note that in the THELEP trial, irrespective of the regimens, 9% of the patients harbored persisters. It could be that the regimen with rifampin, clofazimine and dapsone may be of a bactericidal but not of a sterilizing nature, since the relapses may be due mainly to dormant persisters among the population of bacilli, which may be in a stationary phase of growth after the initial killing of actively growing forms in the initial intensive phase. An important finding in the present study is that the relapses were due to drug-sensitive organisms.
If persisters which are drug sensitive are believed to cause relapse it will not be surprising if we encounter relapse beyond 5 years or more after stopping treatment due to a long drawn out incubation period (3).
In the present report two relapses were noticed among the 51 cases attending the clinic for over 15 years from the start of treatment. The number may be small but it is important to realize that it may be the beginning of the recognition of a major problem.
Acknowledgment. We are grateful to the Director, Central Leprosy Teaching and Research Institute, Chingleput, Tamil Nadu, India for permitting us to do the mouse foot pad experiment for our patients. We are also grateful to our nursing staff for valuable assistance in the clinical work, Mrs. Sivagamasundari for laboratory work, and Mr. R. S. Sen for secretarial assistance.
REFERENCES
1. Almeida, J. G. How effective is monthly rifampin? (Letter) Int. J. Lepr. 60(1992)81-82.
2. Constant-Desportes, M., Guelpa-Lauras, C.-C, C arolina, J.-C, Leoture, A., G rosset, J.-H. and Sansarricq, H. A case of relapse with drug-susceptible M. leprae after multidrug therapy. Int. J. Lepr. 59(1991)242-247.
3. Grosset, J.-H., Guelpa-Lauras, C.-C, Bobin, P., Brucker, G., Cartel, J.-L., Constant-Desportes, M., Flageul, B., Frederic, M., Guillaume, J.-C. and Millan, J. Study of 39 documented relapses of multibacillary leprosy leprosy after treatment with rifampin. Int. J. Lepr. 57(2989) 607-614.
4. Hastings, R. C. and Chehl, S. K.. Chemotherapy of leprosy in multibacillary nude mice. Indian J. Lepr. 63(1991)350-355.
5. Subcommittee on Clinical Trials of the Chemotherapy of Leprosy (THELEP) Scientific Working Group of the UNDP/World Bank/ WHO Special Programme for Research and Training in Tropical Diseases. Persisting Mycobacterium leprae among THELEP trial patients in Bamako and Chingleput. Lepr. Rev. 5(1987)325-337.
6. WHO Study Group. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. M.D., Dip.Lep., Deputy Director;Tuberculosis Research Centre, Madras 600031, India
2. M.Sc, Research Officer, Bacteriology; Tuberculosis Research Centre, Madras 600031, India.
3. B.Sc, B.G.L., Senior Technical Officer, Statistics; Tuberculosis Research Centre, Madras 600031, India.
4. M.D., Director, Tuberculosis Research Centre, Madras 600031, India.
Reprint requests to Dr. Prabhakar.
Received for publication on 11 January 1995.
Accepted for publication on 9 March 1995.