- Volume 63 , Number 3
- Page: 409–16
Intraoperative electroneurodiagnostics to detect a second granuloma in the cubital area of median nerves affected by leprosy: a new approach to prevent incomplete surgery
ABSTRACT
A recent work reports on the necessity to localize the most proximal site of leprous ulnar neuritis with intraoperative electroneurodiagnostics. In the present study we wanted to verify the applicability of this method on leprous median nerves. In six patients, seven median nerves were exposed at the wrist, all showing a typical leprous granuloma there. Spinal roots C5 to Th1 were then stimulated intraoperatively, evoking efferent mixed nerve compound action potentials (NCAPs) which were registered f rom the nerve's surface. No recordings could be obtained on the granuloma in all patients, neither distally nor shortly proximal f rom it, nor even further central at the forearm's proximal third where the median nerve exits the cubital area. Prior to dissecting the nerves in this precarious region, they were exposed at the arm's distal third, looking inconspicuous in all cases. Recordings could finally be obtained there, and subsequent exposure further proximal showed no increase in amplitude of the NCAPs, but there was a sharp decrease distally. In all cases, subsequent dissection of the cubital area revealed a second leprous granuloma extending variably f rom the distal third of the arm to the two heads of the pronator teres muscle, requiring microsurgical release. Intraoperative spinal root stimulation is an effective method to detect a second leprous granuloma and to avoid incomplète surgery in median nerves affected by leprosy.RÉSUMÉ
Un travail récent rapporte la nécessité de localiser le site le plus proximal des névrites cubitales lércuses par électroncurodiagnostic peropératoire. Dans la présente étude, nous avons voulu vérifier l'applicabilité de cette méthode sur des nerfs médians lépreux. Chez six patients, sept nerfs médians ont été exposés au poignet, tous montrant à cet endroit un granulome lépreux typique. Les racines rachidiennes C5 à Dl ont alors été stimulées en peropératoire, provoquant des potentiels d'action nerveux mixtes efférents qui étaient enregistrés à la surface du nerf. Aucun enregistrement n'a pu être obtenu sur le granulome chez aucun patient, ni distalement, ni au niveau proximal à courte distance de celui-ci, ni même à un niveau plus central au tiers proximal de l'avant-bras où le nerf médian quitte la région cubitale. Avant de disséquer les nerfs dans cette région délicate, ils ont été exposés au tiers distal du bras, et ne semblaient suspects dans aucun cas. Des enregistrements ont finalement pu être obtenus là, et une exposition ultérieure à un niveau plus proximal n'a pas montré d'augmentation d'amplitude des potentiels d'action nerveux, mais il y avait une forte diminution au niveau distal. Dans tous les cas, une dissection ultérieure de la région cubitale a montré un second granulome lépreux s'étendant de manière variable du tiers distal du bras aux deux têtes du muscle rond pronateur, ce qui a nécessité une libération microchirurgicale. La stimulation rachidienne peropératoire est une méthode efficace pour détecter un second granulome lépreux et éviter une chirurgie incomplète des nerfs médians affectés par la lèpre.RESUMEN
Recientemente señalamos la necesidad de localizar el sitio más proximal de la neuritis ulnar leprosa mediante el clectroneurodiagnóstico intraoperativo. En el presente trabajo quisimos verificar la aplicación de este método en el estudio de los nervios medianos afectados por la lepra. Para esto se expusieron 7 nervios medianos en la muñeca de 6 pacientes; en todos los casos se observó la presencia de un típico granuloma leproso. Después de estimular intraoperativamente las raíces C5 a Th1 de estos nervios, se registraron los potenciales mixtos de acción (NCAPs) producidos en los nervios eferentes. No se pudieron obtener registros de los nervios a nivel de los granulomas ni distalmcnte ni proximalmentc a ellos, ni siquiera en el tercio proximal al antebrazo donde el nervio mediano sale del área cubital. Antes de disectar los nervios en esta precaria región, se intentó su exposición a nivel del tercio distal del brazo, siendo inconspicuos en todos los casos. Sin embargo, ahí sí se pudieron obtener registros. La exposición subsecuente, todavía más proximal, no mostró incremento en la amplitud de los NCAPs, por el contrario, distalmcnte hubo una marcada disminución de la amplitud de los registros. En todos los casos, la disección subsecuente del área cubital reveló un segundo granuloma leproso con extensión variable del tercio distal del brazo a las dos cabezas del músculo pronator, requiriendo su liberación microquirúrgica. La estimulación intraoperativa de la raíz espinal es un método efectivo para detectar un segundo granuloma leproso y para evitar la cirugía incompleta de los nervios medianos afectados por la lepra.It is estimated that between 10 (19) and 15 (1,22) million people suffer from leprosy worldwide. Thirty (1,16,19) to 60 percent (9,22) have peripheral neuropathies. Surgery on nerves is performed to relieve pain and to restore sensory and motor functions. Poor results following neurolysis in patients suffering from borderline leprosy have been reported (8). A recent publication (23) showed that in 10 ulnar nerves affected by borderline leprosy, the lesion reached farther proximally than the macroscopically affected (thickened) nerve segments. These findings strengthened the hypothesis that one reason for unsuccessful surgery might be insufficient interventions (23), commonly restricted to the obviously damaged part of the leprous granuloma of the ulnar nerve. In the present study, we wanted to verify if detection of the most proximal site of a lesion in median nerves with spinal root stimulation (SRS) (10,14,15) would show similar results as in ulnar nerves.
Patients and Methods
Six patients (four males, two females, age 11 to 47 years; one patient had both upper limbs affected) suffering from borderline leprosy with involvement of the median nerves were included in this study (The Table). All patients had been medically treated according to World Health Organization (WHO) regulations and were involved in this study when improvement of their sensorimotor disorders could no longer be expected. The criteria for case selection was the persistence of neurofunctional deficiency in the affected limb without any signs of recovery for more than 3 months after medication. Motor and sensory status were precisely registered and will be published in a follow-up study to verify the merit of the method described in this paper.
Prior to surgery, the patients underwent conventional nerve conduction velocity studies. The median nerve was stimulated transcutaneously at the wrist, below the elbow, at the axilla, and at the Erb's point (between the clavicle and the trapezius muscle). Muscle action potentials were registered from the thenar eminence with surface electrodes or, if recordings remained absent, with needle electrodes inserted into the abductor pollicis brevis muscle. Since the function of the median forearm muscles seemed normal in all patients, electromyograph (EMG) testing had not been performed.
Anesthesia was induced with ketamine (3 mg/kg i.v.). Patients were relaxed with pancuronium (0.1 mg/kg) and intubated. Artificial respiration was maintained with oxygen (60%)/nitrous oxide (40%). Pancuronium was re-administered during surgery because muscle action potentials may be transmitted to the surface of peripheral nerves surrounded by contracting muscles fibers (i.e., volume conduction). Recordings were performed when full relaxation was achieved in order to avoid the possibility of falsely regarding a volume conducted muscle compound action potential as a nerve compound action potential.
Surgery began with exposure of the median nerve at the wrist. The spinal roots C5 to Th1 then were stimulated with two, silver, plate electrodes glued with collodium onto the skin over the processus spinosus of the vertebrae C4 (cathode) and Th2 (anode), which were connected to the output of a Digitimer D180 electrical stimulator (Fig. 1). To enable investigation of the ulnar nerve as well, a third electrode was glued over the processus spinosus C7 to stimulate spinal roots C8 and Th1(Fig. 2). This was beneficial in comparing the amplitude, duration and shape of ulnar nerve compound action potentials (NCAPs) with the recordings of the median nerves. Output levels ranging from 400 to 600 volts were sufficient to achieve supramaximal stimulus.
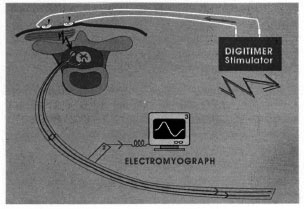
Fig. 1. Schematic of the principle of the stimula-tion technique. ► = Two silver plate electrodes con-nected to the electrical stimulator (1) which generatesthe high-voltage current necessary to stimulate the spi-nal roots;  = efferent mixed (orthodromic motor,antidromic sensory) nerve compound action potential; 2 = bipolar electrode placed on surface of the exposedperipheral nerve to register NCAP; 3 = electromyograph which records the NCAP
= efferent mixed (orthodromic motor,antidromic sensory) nerve compound action potential; 2 = bipolar electrode placed on surface of the exposedperipheral nerve to register NCAP; 3 = electromyograph which records the NCAP
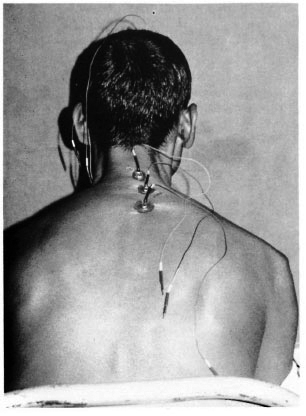
Fig. 2. Silver plate electrodes glued onto a patient preoperatively above the processus spinosus C4, C7 and Th2.
On SRS, efferent NCAPs were evoked. These mixed NCAPs (orthodromic efferent motor and antidromic efferent sensory) were registered with a bipolar sterling silver wire electrode (length: 50 mm, diameter: 1 mm) along the nerve's surface (Fig. 3). To record the NCAPs, we used a Medelec Neurostar MS92I3 electromyograph connected with its external trigger input to the D180. Together with repeated stimulations and recordings, the electrode was moved proximally until amplitudes of the registered NCAPs showed no further increase. If necessary, the initial skin incisions at the wrist were extended proximally up to the upper arm (Fig. 4). In accordance with the ulnar nerve study (23), we considered the most proximal site of the lesion to be the site where amplitudes reached a maximum without showing any increase further proximal.

Fig. 3. Left upper limb of patient M.T. This close-up view of the distal upper arm shows the exposedmedian nerve as it enters into the cubital area; up = proximal, down = distal. ▲ = bipolar, sterling-silverwire electrode placed on surface of the median nerve;
 = common flexor head;
= common flexor head;  = plastic sheet placedunder nerve during measurement to avoid registration of the stimulus current often regarded as an artifact bythe ADC. Note consecutive thickening of the nerve from proximal to distal, indicating the beginning of the second leprous granuloma.
= plastic sheet placedunder nerve during measurement to avoid registration of the stimulus current often regarded as an artifact bythe ADC. Note consecutive thickening of the nerve from proximal to distal, indicating the beginning of the second leprous granuloma.
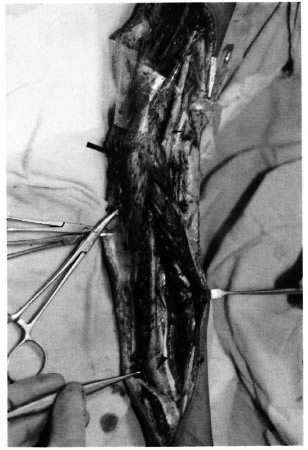
Fig. 4. Upper limb of patient M.T., showing theexposed median nerve at the upper arm and forearm;up = proximal, down = distal, right = ventral, left =dorsal. Note thickening of the median nerve from proximal to distal at the arm ( ), regaining of normal shapeat the proximal third at the forearm where it exits the cubital area (
), regaining of normal shapeat the proximal third at the forearm where it exits the cubital area ( ), and rethickening in the middle of the forearm toward the wrist (▼).
), and rethickening in the middle of the forearm toward the wrist (▼).  = medial epicondyle.
= medial epicondyle.
RESULTS
Preoperative conventional NCV. A complete conduction block was found at the wrist in five cases. In the two remaining cases, muscle action potentials could only be recorded with a needle electrode inserted into the abductor pollicis brevis muscle. Further investigation of the median nerve proximally to the wrist with a conventional nerve conduction velocity(NCV) study was unsuccessful in these patients (The Table). EMG testing might possibly have shown some abnormalities indicative of a proximal median nerve involvement but was not done.
Intraoperative SRS. After surgical exposure of the wrist, all seven median nerves revealed a macroscopically visible granuloma. Its length ranged from under the carpal ligament to the junction between the middle and distal third of the forearm, the diameter from barely enlarged (patient R.B., borderline lepromatous leprosy) to the thickness of a male index finger (patient B.B., borderline tuberculoid leprosy). Proximally to the granuloma, the nerves looked macroscopically inconspicuous. On SRS, no NCAPs could be recorded at the site of the granuloma nor distally from it. Moreover, in none of the cases were NCAPs registered at the transition from the macroscopically unaffected nerve to the beginning of the granuloma. Surgical exposure further centrally was, therefore, necessary in all cases.
Recordings remained absent even more proximally at the median nerves exit from the cubital area (see Technical limitations on page 413). Prior to performing the precarious exposure in this region, the median nerves were dissected at the junction between the middle and distal third of the arm, where they looked inconspicuous in all cases. Recordings were finally obtained there, and subsequent exposure of the nerves further proximal showed no increase in amplitudes. Dissection distally, however, showed a sharp decrease in amplitudes at the entrance into the cubital area, indicating the beginning of an affected nerve segment (Fig. 5). These findings led us to expose the nerve into the cubital area, revealing a second granuloma extending variably from the distal third of the arm to the two heads of the pronator teres muscle in all seven nerves (Fig. 4). All seven second granulomas were smaller in length and diameter compared to the corresponding distal granulomas at the forearm.
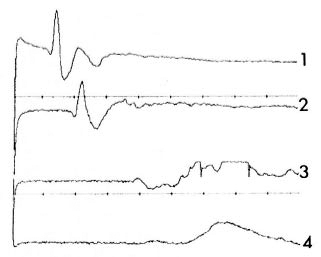
Fig. 5. Recordings from four NCAPs registeredfrom right median nerve of patient A.M. on transcutaneous electrical stimulation of spinal roots C5 and Th1 . Time = 5ms/div; amplification = 100 µ V/div; distance between each registration site = 3 cm. 1 = NCAP registered at the most proximal site of lesion atthe transition between middle and distal third of the arm; 4 = NCAP registered at entrance into the cubitalarea. Note sharp decrease of amplitude (from 220 µ V for 1 to 10 µ V for 4).
Surgical release of the nerves began with epincuriotomy at the macroscopically affected nerve segments, the two granulomas. Microscopic investigation showed in all cases varying degrees of fibrosis within the interfascicular epineurium, as expected. However, this characteristic sign of an inflammation could unexpectedly be observed proximal to the granulomas as well, specifically within nerve segments which looked macroscopically inconspicuous. In the proximal granulomas at the cubita, the fibrosis of the interfascicular epineurium reached to the most proximal site of lesion detected at the arm (where amplitudes showed no further increase) and extended distally to the end of the granuloma, abruptly ending there. In the distal granulomas at the wrist, the fibrosis extended variably from the distal end of the macroscopically visible swelling under the carpal ligament (as expected) centrally to the junction between the proximal and middle third of the forearm, reaching (unexpectedly) within macroscopically inconspicuous segments as well. It should also be pointed out that along a small nerve segment (between 5 and 10 cm) located immediately after the nerve's exit of the cubital channel (the two heads of the pronator teres muscle), the epifascicular and interfascicular epineurium of all seven nerves were free of fibrosis. This means that in all cases, a small nerve segment between the two granulomas had remained unaffected. It should also be mentioned that the fibrosis of the proximal granulomas was milder in all cases compared to the distal granulomas.
Microsurgical interfascicular neurolysis was performed on all patients at the two leprous granulomas and within the nerve segments proximally to them, showing fibrosis of the interfascicular epincurium. Five patients reported pain relief and/or improvement of sensitivity within 2 weeks after the intervention. The sixth patient showed no immediate improvement, most probably due to the long duration of the disease (7 years) (The Table).
DISCUSSION
Technical limitations. Most available electromyographs are not designed fcr intraoperative electroncurodiagnostic measurements. This fact concerns a) exclusion of electrical interferences generated in the operating theater which distort or suppress the measurement procedure and b) the extreme difference of voltage existing between the stimulus intensity (up to 750 volts) and the amplitude of the NCAP (sometimes below 20 microvolts). This difference may reach values around 107 (stimulus is 107 times higher than amplitude of NCAP) and is of crucial importance because most of the electromyograph's inbuilt analog/digital converters (ADC) are not set to accept extremely strong trigger stimuli. If a stimulus is too intensive compared to the amplitude of the recorded NCAP, the ADC regards this stimulus as artifact and omits (rejects) the measurement. With our electromyograph, this phenomenon occurred with amplifications of 50 µ V/div or higher. Therefore, an absent recording with the gain (amplification) set at 50 µ V/div could not be regarded as a total conduction block, i.e., total nerve damage.
Furthermore, the amplitude of a NCAP registered from the nerve's surface not only depends on the number of functioning fibers but also on the thickness of the tissue surrounding the conducting axons. The leprous granuloma consists, among other things, of a postinflammatory fibrosis of the interfascicular and epifascicular epineurium. These structures act as biological insulators, hereby reducing the amplitude of the NCAP.
Both factors, artifact rejection and fibrosis, reduce the measurability of a NCAP from the nerve's surface and have to be understood in order to avoid misinterpretation of absent recordings. This also explains the lack of clinical correlation between the existence of a second granuloma at the cubital area and the absence of corresponding clinical disorders, since the function of the forearm muscles innervated by the median nerve was not visibly disturbed.
Although this technical restriction limited the interpretability of our measurements, the observed relative change of the NCAP amplitudes were so distinct (between 200 µ V and 300 µ V on healthy nerve segments to the still recordable 10 µ V on the damaged parts; Fig. 5) that detection of the lesion's most proximal site was definitely possible. For more precise measurements requiring higher amplifications, we recommend the use of electromyographs with disconnectible artifact rejecters.
Intraoperative electroneurodiagnostics versus conventional NCV studies. Conventional surface NCV studies in leprosy patients are mainly used for the following two purposes: a) early detection of the disease and b) verification of the surgical success (2.4-6,17,20 ). We believe that conventional NCV studies are less reliable in localizing the most proximal site and extent of nerve damage than SRS because: a) conventional surface NCV recordings are influenced by the thickness of the tissues between nerve and electrode. This makes a reliable and useful recording of NCAPs along the entire course of the nerve within the upper limb almost impossible. The amplitude of the NCAP, however, is essential to evaluate the number of functioning fibers at any site of the nerve; b) conventional NCV may fail to show abnormal conduction time if some of the fastest conducting fibers remain unaffected; c) in conventional NCV recordings errors in measurement of nerve segment length may occur, especially when there are short distances between stimulation and recording electrodes. With SRS, in contrast, the nerve is stimulated from its most proximal site, the roots, providing the longest possible measuring distance; d) the most often existing conduction block of the median nerve at the wrist makes any investigation further proximally and, subsequently, also the localization of the most proximal site of lesion with conventional surface NCV studies very difficult.
The first two points are probably responsible for the failure to determine the proximal extent of the lesion by McLeod, et al. (11). These authors used a modified NCV technique (13) to determine the correct site (i.e. certainly unaffected) for homologous nerve transplants (11,12) in leprous neuritis. They stated that they managed to ". . . localize an upper level of a lesion . . ." (11), but found that ". . . even though nerve conduction studies were frequently within the normal range proximal to the excised portion of nerve, subsequent pathological examination indicated that the proximal segment was (still) affected by disease" (11).
General aspects and surgical implications. The reason for determining the full extent of a lesion in leprous neuritis is obvious: if an affected segment of the nerve remains unreleased during surgery, regeneration would be reduced or possibly not occur at all. Turkof, et al. (23) have demonstrated in 10 cases of leprous ulnar neuritis that incomplete intervention can be avoided with the use of intraoperative electroneurodiagnostics. In fact, these authors had simply confirmed Sir Sydney Sunderland (21) with electrophysiological methods. This well-known author had already stated in 1973 that leprous nerves can be functionally disturbed without requiring obvious enlargements (21). Despite these early findings, many authors (3,4, 7, 8, 16-18, 22) restricted their interventions to the obviously affected segment of the leprous granuloma at the wrist. According to our results, it is most probable that many of their cases had a second granuloma at the cubital area which remained unreleased.
The pilot study of the 10 ulnar nerves (23) took on an additional dimension when applied to median nerves: the search for the most proximal site of a lesion in the present study led to the detection of a second granuloma which was not previously diagnosed by conventional NCV studies.
It has to be pointed out that the described method should not be regarded as a surgical guideline. Our measurements showed us where the disease (lesion) started. Epineuriotomy was, therefore, performed up to this area, but any further step was decided according to the situs observed through the operative microscope. Interfascicular microsurgical neurolysis should only be performed if the interfascicular epincurium is fibrosed. Our findings indicate the necessity of cooperation between leprologist, neurophysiologists and plastic (hand) surgeons.
We conclude that a) median nerves affected by borderline leprosy may show a second leprous granuloma in the cubital area hardly detectable by conventional NCV studies, b) detection of this second granuloma and the most proximal site of the lesion is imperative in order to avoid incomplete surgery, and c) intraoperative electroneurodiagnostics (SRS) are most adequate to detect the most proximal site of leprous median neuritis.
Acknowledgment. This study was financially supported by scientific funds from the Medical School of Vienna, the Surgical University Clinic of Vienna, Zeneca Inc., and the Ludwig Boltzmann Institute for Experimental Plastic Surgery, Vienna.
The authors wish to thank Dr. Kharkar, Bombay, India, for his friendly cooperation in organizing the leprosy patients. The authors also wish to thank Dr. (Mrs.) Pravina U. Shah, K.E.M. Hospital, Bombay, India, for her friendly cooperation in providing the measurement facilities and her excellent, skilled staff members.
REFERENCES
1. Antia, N. H., Enna, C. D. and Behman, M. D. The Surgical Management of Deformities in Leprosy and other Peripheral Neuropathies. Bombay: Oxford University Press, 1992, pp. 1-17.
2. Antia, N. H., Pandya, S. S. and Dastur, D. K. Nerves in the arm in Leprosy. I. Clinical, electrodiagnostics and operative aspects. Int. J. Lepr. 38(1970)12-29.
3. Bourrel, P. Place de la chirurgie dans le traitement et la réhabilitation des lépreux. Chirugie 108(1982)744-752.
4. Carayon, A. Décompression chirurgicale des névrites lépreuses I. Bases anatomo-cliniques et physio-pathologiques. Chirurgie 110(1984)219-224.
5. Charosky, C. B. Neuropathies in Hansen's discase. Int. J. Lepr. 51(1983)576-586.
6. Dastur, D. K. The nervous system in leprosy. In: Scientific Approaches to Clinical-Neurology. Goldensohn, E. and Appel, S., cds. Philadelphia: Lea & Febiger, 1977, pp. 1463-1493.
7. Dastur, D. K. Pathology and pathogenesis of predilictive sites of nerve damage in leprous neuritis. Neurosurg. Rev. 6(1983)139-152.
8. Giraudeau, P., Corbeille, R. and Nerot, C. Quinze années de chirurgie du nerf lépreux. Résultats de 644 interventions en fonction des formes évolutives. Acta Leprol. (Genève) 86-87(1982)227-234.
9. Kumar, R. Surgical management of leprous ulnar neuritis. Clin. Orthop. 163(1982)235-242.
10. Marsden, C. D., Merton, P. A. and Morton, H. B. Percutaneous stimulation of spinal cord and brain: pyramidal tract conduction velocities in man. J. Physiol. 328(1982).
11. McLeod, J. G. Hargrave, J. C, Gye, R. S., Pollard, J. D., Walsh, J. C, Little, J. M. and Booth, G. C. Nerve grafting in leprosy. Brain 98(1975)203-212.
12. McLeod, J. G., Hargrave, J. C, Pollard, J. C, Loewenthal, J. and Booth, G. C. Use of immunosuppressive agents in human nerve grafting. Lancet 1(1972)647-650.
13. McLeod, J. G., Hargrave, J. C, Walsh, J. C, Booth, G. C, Gye, R. S. and Barron, A. Nerve-conduction studies in leprosy. Int. J. Lepr. 43(1975)21-31.
14. Merton, P. A., Morton, H. B., Hill, D. K. and Marsden, C. D. Scope of a technique for electrical stimulation of human brain, spinal cord, and muscle. Lancet 2(1982)597-600.
15. Mills, K. R. and Murray, N. M. F. Electrical stimulation over the human vertebral column: which neural elements are excited? EEG Clin. Neurophysiol. 63(1986)582-589.
16. Nores, J. M., Merle, F., Redondo, A., Vernerey, C. and Gentilini, M. Surgical management of leprous neuritis: results of 114 operations. Ann. Trop. Med. Parasitol. 83(1989)163-165.
17. Pandya, J. N. Surgical decompression of nerves in leprosy, an attempt at prevention of deformity; a clinical, electrophysiologic, histopathologic and surgical study. Int. J. Lepr. 46(1976)47-55.
18. Pereira, J. H., Palande, D. D. and Gschmeissner, S. E. Mycobacteria in nerve trunks of long-term treated leprosy patients. Lepr. Rev. 62(1991)134-142.
19. Pereira, J. H., Palande, D. D., Subramanian, A., Narayanakumar, T. S., Curtis, J. and Turk, J. L. Denatured autologous muscle graft in leprosy. Lancet 338(1991)1239-1240.
20. Sabin, T. D. and Swift, T. R. Leprosy. In: Peripheral Neuropathy. Vol. 2. Dyck, P. J., Thomas, P. K.., Lambert, E. H. and Bunge, R., eds. Philadelphia: W. B. Saunders, 1984, pp. 1975-1977.
21. Sunderland, S. The internal anatomy of nerve trunks in relation to the neural lesions of leprosy - observations on pathology, symptomatology and treatment. Brain 96(1973)865-888.
22. Taraporvala, J. C. Surgical decompression for leprous neuritis. Indian J. Orthopaed. 10(1976)1-6.
23. Turkof, E., Tambwekar, S., Mansukhani, K., Millesi, H. and Mayr, N. Intraoperative spinal root stimulation to detect most proximal site of leprous ulnar neuritis. Lancet 343(1994)1604-1605.
1. M.D.; Department of Plastic and Reconstructive Surgery, Surgical University Clinic of Vienna, and the Ludwig Boltzmann Institute for Experimental Plastic Surgery, Vienna, Wahringer Gürtel 18-20, 1090 Vienna, Austria.
2. Hon. Prof.; M.D., F.R.C.S., Department of Plastic and Reconstructive Surgery;
3. M.D., Department of Neurology, K. E. M. Hospital, Parel, Bombay 400012, India.
4. Prof.; M.D., Department of Plastic and Reconstructive Surgery, Surgical University Clinic of Vienna, and the Ludwig Boltzmann Institute for Experimental Plastic Surgery, Vienna, Wahringer Gürtel 18-20, 1090 Vienna, Austria.
5. M.D., Associate Professor, Neurological University Clinic of Vienna, Wahringer Gürtel 18-20, 1090 Vienna, Austria.
Reprint requests to Dr. Turkof, Abteilung fur Plastische und Rekonstruktive Chirurgie, Klinik fur Chirurgie - Allgemeines Krankenhaus der Stadt Wien, Wahringer Gürtel 18-20, 1090 Wien, Austria. FAX 43-1-40400-6988.
Received for publication on 4 December 1994
Accepted for publication in revised form on 14 June 1995.