- Volume 63 , Number 3
- Page: 430–47
The immunology of leprosy; unraveling an enigma
A new look at an ancient disease an historical perspective
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. The disease has a broad spectrum of clinical manifestations from the tuberculoid form, with a low bacillary load and good prognosis, to the lepromatous form, which is multibacillary and may lead to irreversible physical deformities and extensive damage to the peripheral nerves. Apart from the physical corollaries of leprosy, the nature and course of the disease cause considerable psychological distress that is exacerbated by a trenchant social stigma associated with the condition.
The beginning of a scientific history of leprosy may be placed toward the end of the last century when, in 1873, Hansen, a Norwegian specialist in leprosy, identified the M. leprae bacilli in lepromatous nodules. However, a clinical appreciation of the disease dates back to ancient times, with the earliest descriptions of leprosy being found in Indian manuscripts dated circa 600 B.C. An Egyptian skeleton dating back to the 2nd century B.C. shows the unequivocal signs of the disease, demonstrating the antiquity of this devastating condition.
Despite the extraordinary advances in the clinical and basic biological sciences witnessed in this century, leprosy has survived into modernity. An increased understanding of the infectious diseases and a steady rise in the standard of living in the West, with enlightenment to the practices of good hygiene and the development of sterile conditions in medical and surgical practice, have inevitably led to the virtual eradication of the disease in Europe and North America, while it remains a major concern in developing countries. The major burden of the disease is borne by tropical and subtropical countries, of which India, South America, Central Africa and Southeast Asia are the most affected. India has the highest prevalence of leprosy at an estimated 3 million cases in 1991. Endemic pockets have also persisted in eastern Europe and in the southern and coastal states of the U.S.A. where the disease is most prevalent among immigrants and in the Cajun population of the state of Louisiana.
Thus, leprosy remains a major health problem in the developing world with an estimated 12 million cases worldwide, making it one of the major infectious diseases as classified by the World Health Organization (WHO) and a topic of intense research. Despite much attention, leprosy continues to be one of the major unconquered infectious diseases of the world whose secrets have only recently begun to unravel under the unrelenting pressure of scientific scrutiny.
Clinical and histological spectrum in leprosy
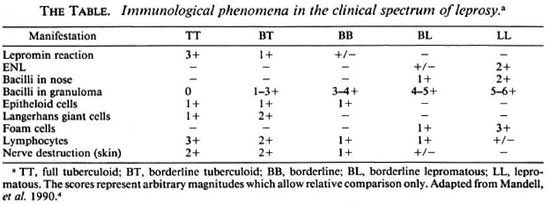
Consideration of the range of clinical manifestations and their attendant pathologies in M. leprae infection provides the first indications to the immunological, genetic and microbiological forces that determine the course of the disease. This spectrum of disease activity is best classified by the five-group system of Ridley and Jopling,1 in which a progression from the most limited form of disease to generalized disease is as follows: TT, full tuberculoid → BT, borderline tuberculoid → BB, borderline → BL, borderline lepromatous → LL, full lepromatous. Patients classified within the borderline (BB) region of the spectrum tend to be clinically unstable and usually migrate toward the tuberculoid or lepromatous poles, which tend to be regions of greater disease stability.
Lesions of TT and BT leprosy typically present as large, well-defined, erythematous plaques that are anesthetic and usually hairless. Damage to peripheral nerves is usually confined to one or two which may be visibly swollen. Biopsy specimens of skin lesions demonstrate the presence of Langerhanstype cells and extensive lymphocytic infiltration of granulomas. T lymphocytes of the CD4+ subset are most abundant in the central region of the granulomas with CD8 + T lymphocytes present as a mantle around the epithelioid structures. All of the lymphocytes within the granulomas display an activated phenotype. A characteristic histologic feature of TT leprosy is nerve bundies that are grossly swollen and extensively infiltrated with mononuclear inflammatory cells. Acid-fast bacilli (AFB), if present, are few.2
In BB leprosy, skin manifestations are more numerous, and satellite lesions may be present in the periphery of larger plaques. The lesions are usually irregularly shaped, erythematous plaques or may be annular in form. The histopathology of these lesions are variable, although there is generally ill-defined granuloma formation, fewer lymphocytes and readily demonstrable AFB.
In LL leprosy there is extensive skin involvement that presents commonly as multiple plaques and nodules. The skin becomes progressively thickened with infiltrate, producing the classical leonine facies with loss of eyebrows. Although nerves are diffusely involved by granulomatous inflammation, sensory or motor defecits are less pronounced since a poor cell-mediated immunity (CMI) limits inflammatory structural and functional damage. The predominant cell type is the macrophage, the natural cellular host for M. leprae, which possess "foamy" inclusions as a result of M. leprae infection. Langerhans-type giant cells are absent, lymphocytes are few and epithelioid cell formation is poorly developed. The granulomatous infiltrate within the dermis remains separated from the basal layer of the epidermis by a thin band of stroma called the clear zone. There is a general granulomatous spread throughout the lymphoreticular system involving the lymph nodes, liver and spleen. The paracortical regions of lymph nodes are massively infiltrated with macrophages laden with bacilli which also colonize and destroy the perarteriolar regions of the spleen. In contrast, germinal centers of lymph nodes are surprisingly free of pathology and are sometimes expanded.3
Thus, the clinical spectrum of disease is closely mirrored by the immunologic status of the host which, in turn, seems to determine the course of the disease (The Table).
This complex interplay uncovers fundamental issues in the immunomodulation of leprosy. Does the state of host immunity determine which arms of the immune system are recruited in combating infection or does M. leprae manipulate the immune system by activating arms of the immune system that do not contribute to protective immunity? Further, if both host and M. leprae contribute to the state of immune activation then what are the factors that determine the immunological and, ultimately, the clinical outcome? Finally, and inclusively, what factors contribute to the clinical spectrum of leprosy?
Multifactorial etiology evidence from epidemiology
If host genetic and immunologic factors contribute to the clinical outcome in leprosy, then it would be expected that genetically different populations would respond differently to M. leprae infection, skewing the distribution over the clinical spectrum. There is some epidemiological support for this view. Although leprosy occurs in all races, it is well known that the frequencies of the two polar forms of leprosy, TT and LL, vary considerably, both racially and geographically. About 20% of the known cases in India are LL; whereas the prevalence is 30%-40% in whites and among orientals of Japan, China and Korea. Conversely, TT leprosy may constitute up to 90% of the total prevalence in Central Africa. These findings suggest that racial genetic differences influence the pathology of leprosy, but specific investigations of genetic association have produced equivocal results. The concordance of leprosy in monozygotic twins is too low to suggest a strong genetic involvement; whereas investigations of the human leukocyte antigen (HLA) distribution in LL patients does not yield consistent associations.4
Recent advances in our understanding of the functions and dynamics of the immune system, brought about by the revolution in molecular biology, has allowed detailed investigation of the cellular and molecular aspects of the immunology of leprosy, yielding many insights into the complex world of host-parasite interaction. Basic advances in parasitology and the recognition of its vital relation to immunomodulation have produced the basic infrastructure which promises to lead to an exciting new paradigm in which to understand leprosy.
An Immunological Anatomy of Leprosy; Pieces of the Puzzle Leprosy and immune reactivity
The most striking contrast between the immunological poles of leprosy is the profound and M. leprae- specific unresponsiveness, or anergy, of T cells in LL leprosy as opposed to the strong T-cell proliferative responses seen in TT leprosy. There is much debate as to the nature of the T anergic state and its induction, although several emergent models appear to be gaining support. The most prevalent model focuses on the newly characterized Th ½-dichotomy in the production of protective or suppressive cytokine profiles in response to antigen-driven activation. Another model postulates that a Ts-subset is activated by suppressor epitopes of M. leprae which then specifically switch off M. leprae -reactive, protective T-cell subsets. These two theories are not mutually exclusive since cytokines secreted by Ts-cells may lead to specific anergy.
Cytokines: losing the balance?
Cytokines have taken center stage in the study of the immunology of parasitic infections since the demonstration of an association between the Th-½ dichotomy and protective immunity. Immunity to various protozoan, bacterial and helminthic infections have been shown to be associated with the preferential induction of a Th1 or Th2 response.5,6 Numerous investigators have reported distinct patterns of cytokine production in specific parasitic infections. In the parasitic infection of macrophages it is generally observed that Th1 responses, producing gamma-interferon (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-2 (IL-2), are protective; whereas TH2 responses, producing IL-4, IL-5, IL-6 and IL-10, are nonprotective. These patterns of immunity were, until recently, observed only in murine models whose relevance to human immunity was unclear. With the advent of sensitive new techniques for detecting cytokine production in human parasitic disease, such as the polymerase chain reaction (PCR) and immunofluoresence, these subsets of CD4+ T cells became central to considerations of protection and pathology in human disease.
It is thought that the Th 1 and Th2 subsets arise from a common precursor, the Th0-cell.The ThO subset is a mature and activated CD4+ population whose cytokine profile is a combination of Th1 and Th2 phenotypes which differentiates into the distinct Th1/Th2 subsets on appropriate stimulation. The nature of this activation and differentiation process is poorly understood but is thought to be influenced by the type of antigen-presenting cell (APC) and by the cytokine milieu at activation. In the mouse, different APC populations are able to selectively induce a Th1 or Th2 response. Antigen presentation by macrophages produce a Th1 response; whereas presentation by B cells induces a Th2 response in a murine system with ovalbumin (OVA) as antigen.8 Thus, it is thought that Th1 induction mobilizes CMI; whereas induction of a Th2 response mobilizes antibody production by providing cytokine help for B-cell activation and immunoglobulin class switching.
Studies of cytokine profiles in leprosy lesions reveal a similar dichotomy between the polar forms of disease. By amplifying reverse-transcribed cDNA from mRNA isolated from lesion biopsy samples and detecting the message using specific cytokine primers, it is possible to detect cytokines at a very high sensitivity. Using this method, workers have reported differences in cytokine production between tuberculoid and lepromatous leprosy.9 Cytokine mRNAs predominantly produced by macrophages (IL-1β, TNF-α, GM-CSF, TGF-β, IL-6) were more abundant in the tuberculoid than in the lepromatous lesions. Further, IL-2 and IFN-γ were abundant in the tuberculoid form; whereas these cytokines were virtually absent in lepromatous lesions. In the latter there was a more prominent expression of IL-4 and IL-10 messages. Thus, lymphokine mRNAs characteristic of the Th1 subset correlated with a good CMI or delayed-type hypersensitivity (DTH) reaction characteristic of spontaneously resolving tuberculoid lesions; whereas a Th2 profile correlated with progressively degenerative disease leading to the profound anergy of lepromatous leprosy.
Other studies have demonstrated the importance of the cytokine milieu to disease activity and outcome. Injection of Th1 cytokines, such as IL-2, into LL lesions produces clear signs of increased CMI accompanied by a significant increase in the degradation of M. leprae. 10 Th1 cytokines also have been shown to confer protection in other mycobacterial infections. A murine knockout model lacking the IFN-γ gene is unable to resolve an otherwise transient M. tuberculosis infection.
The disease spectrum of leprosy has striking corollaries with another parasitic infection that is tropic for macrophages. Clinical presentation in leishmaniasis, caused by infection with Leishmania (a protozoan parasite), can range from self-healing cutaneous to uncontrolled, diffuse cutaneous disease, from mild to highly destructive mucosal disease, and from subclinical to fatal systemic visceral disease.11 Patients with active visceral leishmaniasis lack Leishmania- specific DTH responses during acute disease. In contrast, patients with nonfatal cutaneous leishmaniasis have strong DTH. Recent studies using murine models of leishmaniasis have revealed that CD4 + T-cell subsets are crucial in determining disease outcome. A Th1-type cytokine response is associated with protection; a Th2 response is related to susceptibility to disease. This, along with the cytokine patterns observed in leprosy, suggests that infection of the macrophage is a key factor in determining the nature of immune mobilization, allowing the induction of a protective Th1 response and driving disease activity toward the tuberculoid pole. In lepromatous disease there is an abberant activation of the immune response, inducing a Th2 population of T cells which do not possess the relevant effector mechanisms for clearing infection of macrophages. Thus, the role of the macrophage in clearing infection and the means by which parasites persist within them must be considered in any attempt to explain the immune phenomena of leprosy.
The parasitized macrophage. The macrophage is well established as the major reservoir of mycobacterial infection. Although Schwann cells and keratinocytes, among other cell populations, have been shown to harbor M. leprae, 12 the macrophage carries by far the largest burden in multibacillary disease. Such parasitized macrophages are also evident as the principal colonizing population, within areas normally occupied by T cells, of the lymphoreticular network and features prominently in lepromatous granulomas. Since macrophages have been demonstrated to be crucial immunomodulators in other parasitic infections, it seems likely that such a role may be relevant in determining the parameters of the Th1/Th2 subset induction in leprosy.
Macrophages subserve two major functions within the immune system: a) to present antigen via MHC Class I and II molecules to T cells of the CD8+ and CD4 + subsets, respectively, and b) to phagocytose and destroy pathogens via nonspecific effector mechanisms.13,14 The macrophage as an APC is important in inducing CMI by the activation of T-cell subsets in an MHC-restricted manner (Fig. 1). As already mentioned, macrophages induce a Th1 subset of CD4+ cells; whereas B cells, as APCs, tend to induce Th2 CD4+ cells. Since Th1 cytokines such as IFN-7 and TNF-a are stimulatory to macrophages, such a response would be appropriate in clearing M. leprae infection via macrophage cytotoxicity. Indeed, there is a predominance of cytokines typical of macrophage activation in TT lesions where bacillary loads are extremely low, suggesting efficient clearance.9
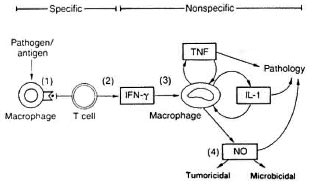
Fig 1. The dual role of macrophages in antigen presentation and microbicidal function.
The principal product of macrophage activation directed toward intracellular parasites is nitric oxide (NO).13 Nitric oxide is the end product of L-arginine metabolism, and its biosynthesis is catalyzed by NO synthase (NOS). The parasitic targets of NO action remain unclear although its ability to kill or inhibit the replication of intracellular parasites is well demonstrated. Thus, effective macrophage activation through Th1 induction should allow effective control of M. leprae infection. However, induction of Th2 cytokines, especially IL-10,15 is inhibitory to macrophages and results in a reduction of TNF-α, IL-1 and IL-6 production. Consequently, M. leprae is able to persist and replicate within the macrophage leading to multibacillary disease in LL leprosy.
Host resistance genes
It has long been believed that the ability of the host to resist infection is strongly influenced by genetic factors. The clinical spectrum of leprosy and the epidemiological heterogeneity, both geographical and ethnic, in the prevalence of polar forms of the disease may be explained by genetic differences in host resistance. Although the specific genes involved have not been identified, recent studies suggest that the basis for this genetic difference resides in the differential capacity of host macrophages to kill phagocytosed bacilli.
Bcg and macrophage activation. The importance of macrophages in mycobacterial infections has been consolidated recently by the demonstration that the murine bcg gene, responsible for innate resistance or susceptibility to mycobacterial infection, is expressed by mature macrophages.16 The gene, present on chromosome 1, exists in two allelic forms (begs and begr) that confer susceptibility and protection, respectively, in the early stage of infection with M. lepraemurium. The mechanism by which bcgr macrophages exert enhanced cytocidal or cytostatic activity is unknown, but they appear superior to bcgs macrophages in the expression of surface markers associated with activation and in the production of toxic nitrogen and oxygen radicals. The magnitude of both the hexose monophosphate shunt and respiratory burst activity are significantly greater in macrophages of resistant animals to these displayed by susceptible mice, following either infection with BCG or treatment with IFN-γ.
A candidate for the bcg gene, Nramp, has recently been identified in the mouse.17 The expression of this gene is generally restricted to reticuloendothelial tissues and to macrophages in particular. Sequence analysis of the translated Nramp peptide predicts a protein with the characteristics of a membrane-associated transporter. The putative Nramp protein also has a 20-amino acid protein motif, termed the binding-proteindependent transport system inner membrane component signature. This motif is characteristic of ATP binding transporter systems. The nature of the substrate transported by Nramp remains unclear although the structural homology between Crna, a membrane transporter responsible for nitrate import in Aspergillus nidulans (an eukaryotic fungus), and Nramp raises the interesting possibility of a similar role for Nramp in the macrophage.18 Thus, the Nramp transporter could play a role in concentrating NO and its derivatives into the phagolysosomal compartment of macrophages, leading to maximal cytocidal activity and mycobacterial killing. A defect in this pathway would lead to persistence and multiplication of mycobacteria, leading to a multibacillary infection.
Other workers have suggested a signal transduction and transmodulation function for bcg (and the Nramp protein) in the differential activation of macrophages.19 The TNF-α response of activated macrophages is induced preferentially by binding to the extracellular matrix (ECM) components fibrinogen and fibronectin. This integrin-mediated interaction leads to a greater TNF-α response in bcgr than in begs strains of mice. However, significant nitrite production is only induced after administration of IFN-γ as a second costimulatory signal. This response was specific to fibrinogen or fibronectin-stimulated macrophages. These findings implicate Nramp in the transduction pathway for both the TNF-α response and the toxic nitrogenous products of macrophage activation. The presence of numerous protein kinase C phosphorylation sites, as well as an Src (tyrosine kinase) domain, thought to mediate protein-protein interaction in signal transduction, provide strong support for Nramp-mediated differential activation/priming of macrophages, from susceptible and resistant strains, by acting as a costimulatory molecule in integrin-mediated, tyrosine kinase signal transduction pathways. Nramp may also play a part in the amplification of signals in the induction of MHC class II molecules since this is also sensitive to the IFN-γ signal.
Sequencing of the mRNA transcripts of Nramp from begr and bcg s strains of mice reveals aG → A transition in all susceptible strains. This nucleotide change results in a nonconservative Gly to Asp substitution at amino acid position 105. Since this change in the primary structure of Nramp correlates with the ability of macrophages to clear mycobacterial infection in murine models, the allelic differences between bcgr and bcgs 1 provide an insight into the molecular basis to the stark differences in bacillary loads observed in the polar forms of leprosy.
HLA and the T cell: molecular partners in immunomodulation. While the early, macrophage-dependent phase of the host response to M. leprae infection seems to be under the control of the bcg gene,20 the late or T-cell-dependent phase of the response seems to be linked to the HLA locus,21,22 especially the class I and class II regions coding for molecules involved in antigen presentation to HLA-restricted T cells. However, the HLA locus does not seem to confer protection or susceptibility to infection but, rather, determines the subsequent course of disease.
Antigen is presented in the context of HLA class I/I I molecules. These molecules possessa peptide-binding groove in which fragments of antigenic and self proteins are presented for the scrutiny of T cells. The binding characteristics of an HLA molecule is dependent on the topology of the peptidebinding cleft and its electrostatic and biochemical composition.23 Thus, HLA molecules bind peptides with characteristic binding motifs that complement the binding cleft.24 Recent advances in synthetic peptide chemistry have allowed detailed characterization of many of the binding motifs of HLA molecules,25,26 producing valuable insights into the vital T-cell component of immunity against pathogens. Although class I molecules are expressed in virtually all nucleated cells in humans, class II molecules are expressed by specialized APCs of lymphoid origin, such as macrophages, B cells and dendritic cells. Before antigen is presented by HLA molecules it must be processed to produce peptidic fragments suitable for binding to the peptide binding clefts of HLA molecules. Antigens presented by class I molecules are generally derived from endogenous proteins and those produced as a result of viral infection; class II molecules present exogenously derived self and antigenic peptides. The processing pathways allowing differential trafficking of endogenous and exogenous peptides are complex and, as yet, not fully understood. Moreover, there is some overlap in these pathways, allowing endogenous peptides to be presented by class II molecules and vice versa.21 Similarly, T-cell receptors (TCR) that recognize immunodominant peptides in the context of specific restriction elements are expected to have similar configurations and show preferential Vβ-usage.
The immunogenetics of leprosy suggest that HLA genes play an important role in the immunomodulation of pathology. Among individuals susceptible to leprosy, those with HLA-DR3 are more prone to progress to the tuberculoid pole whereas those with HLA-DQ1 develop lepromatous disease.27 Epidemiology indicates that certain HLA genes are more prevalent in endemic regions (HLA-DRw53), suggesting that resistence or susceptibility is, at least in part, immunologically defined.28 A precedent for such a relation is found in studies of protective immunity from severe malaria in endemic regions. Investigators report the association of HLA-B53 with protection, which is demonstrated at the cellular level by CD8+ T-cell-mediated cytotoxicity to the malarial LSA-1 antigen in HLA-B53 + individuals from the endemic regions of Gambia.29 The epitopes of LSA-1 recognized by these T cells conformed to the HLA-B53 binding motif.
The p3-13 (KTIAYDEEARR)30 sequence in the 65-kDa heat shock protein (hsp65) of M. leprae serves as a major immunodominant epitope in HLA-DR17 + individuals by selectively binding to HLA-DR17, the major subset of the DR3 specificity.31The core peptide-binding motif of HLA-DR17 was determined, by truncated peptide analysis, to be eight amino acids in length with an anchor residue at position one, favoring large hydophobic residues (I, L, V), and a second anchor at position three, favoring negatively charged residues (D, E). Epitopes of hspl8 and hsp70, both important T-cell stimulatory proteins in murine M. leprae infection, also conformed to this motif, illustrating the importance of the binding repertoire of HLA molecules in determinant selection and the consequent immunity in leprosy.
Recognition of HLA class II presented peptide is mediated by the TCR. In recognizing mycobacterial hsp presented by HLA-DR17, several key positions affected TCR recognition. Residues 6(A), 7(Y) and 9(E) were seen to be important, and thus probably interact with the CDR3 region of the TCR. The peptide/MHC criteria required for recognition by T cells is reflected in the configuration of their TCRs. In a study investigating Vβ-gene usage in T cells isolated from tuberculoid and lepromatous lesions, the TCR repertoire was significantly skewed in tuberculoid lesions; lepromatous lesions did not show preferential Vβ usage.32 The overrepresentation of the Vβ6 gene family in tuberculoid lesions argues for oligoclonal T-cell activation in tubercuoid leprosy directed at a small number of antigens. Thus, identification of M. leprae antigens recognized by Vβ6-bearing T-cells may lead to characterization of the antigen(s) driving tuberculoid disease.
Heat-shock proteins: immunodominant antigens in M. leprae infection. Heat-shock proteins (hsp) are a highly conserved family of proteins found in both prokaryotic and human cells and are induced in response to cellular stress.33 They are classified by molecular weight and perform their normal cellular functions by associating with and functionally influencing other proteins. Members of the hsp60 and hsp70 families play roles in the folding and unfolding of proteins and in the assembly of oligomeric protein complexes. In addition, hsp70 is involved in antigen translocation and presentation. During stressed conditions, such as elevated temperature, exposure to free radicals and extreme pH, these functions come to the fore and are necessary for cell survival. Not surprisingly, in the stressful environment of the host, microorganisms produce hsp and, hence, they are frequently the dominant antigenic targets for cellular and humoral immunity.
T-cell reactivity to M. leprae hsp correlates well with the clinical and immunological spectrum of leprosy, so that strong CMI responses are observed in tuberculoid patients through too little or no CMI to hsp in lepromatous disease. Various mycobacterial hsp have been shown to elicit strong T-cell proliferative responses in M. leprae -infected individuals, of which hsp65, hsp70 and recently hsp10 are of particular importance in producing vigorous CMI and the generation of immunological memory. 34-36 Fine mapping of hsp T-cell epitopes and their respective restriction elements is allowing a more detailed understanding of the immune response against leprosy and, importantly, providing a suitable setting for the development of subunit vaccines in leprosy.
Thus far, the emphasis of epitope mapping studies has been on the stimulatory function of mycobacterial hsp on T cells. However, the activity of T-suppressor (Ts) cells in the immunomodulation of leprosy has long been documented.37 The contention that the T-cell unresponsiveness seen in lepromatous patients is not due to an absence of reactive T cells but due to active suppression of protective Th cells is supported by numerous studies in which CD4 + and CD8+ T cells from the peripheral blood and skin lesions of LL patients have been shown to specifically suppress mycobacterium-specific T-cell responses in vitro. 38 These T cells express TCR a and /3 chains and are restricted by HLA-DR or HLA-DQ molecules. This Ts-cell population poorly express the costimulatory receptor molecule CD28 and have a reduced capacity to proliferate to M. leprae- specific antigens. In light of the suppressive nature of Th2 cytokines in the murine models of M. leprae infection, it would be reasonable to suppose a role of this subset as a suppressor population in human disease. Although there is some support for a Th2-suppressor population operating in leprosy, this issue remains to be resolved since the precise mechanism for suppression in these systems has not been elucidated.
An exciting new development in the search for antigenic epitopes of M. leprae is the recent characterization of a T-cell-suppressor epitope in M. leprae hsp65.39 Soluble M. leprae antigens were used to screen Ts clones generated from borderline LL patients for proliferation to specific mycobacterial antigens. Using deletion mutants and overlapping synthetic peptides of hsp65, the epitope recognized by this Ts clone was mapped to residues 435-449 of hsp65, with the minimal recognition sequence spanning residues 439-448. The Ts clone was characterized as CD4 + , HLA-DRB1*1503 (DR2). Interestingly, this epitope previously has been reported as a stimulatory epitope in HLA-DR1-restricted individuals, suggesting that host genetic background plays an important part in determining pathology. This is also seen in rodent models of leprosy, and has intriguing similarities to the role of genetic factors in the autoimmune diseases.
The prominence of mycobacterial hsp as immunodominant antigens in leprosy is further demonstrated by the wide range of HLA restrictions of the reactive T cells. The particular importance of HLA-DR molecules in immunomodulation, and hence the progression of disease, has already been alluded to. It is not surprising that HLA-DR is the major element in antigen presentation since it is expressed at much higher densities than HLA-DP or-DQ. However, in most models of antigen presentation, peptide is presented in an allele-specific manner within this isotype. Recently, degeneracy in peptide binding has been demonstrated between HLA-DR molecules allowing particular antigenic peptides to be presented by multiple alleles with subtly different binding motifs.40 Such a mechanism would explain the wide range of allelic restrictions of particular hsp. Hsp65 is presented by HLADR 1, DR2, DR5, DR7 and DR4 expressing APCs;41 whereas other hsp (hsp 18, hsp70) show more allele-specific reactivities. Such panallelic HLA-DR presentation characteristics of hsp65 might explain its predominance, as a suppressor and stimulatory antigen, in T-cell-mediated immunity in leprosy.
Heat-shock proteins, nerve damage and autoimmunity. Nerve destruction is one of the major causes of deformity in leprosy. Within the clinicohistological spectrum of disease, peripheral nerve damage, due to neuritis and resulting in paralysis and anesthesia, is more severe in tuberculoid than in lepromatous disease, presumably due to differences in CMI at the two poles. The mechanism of nerve injury is poorly understood, although Schwann cells are known to be important reservoirs of infection in the multibacillary state and have been demonstrated to express both HLA class I and II molecules on its surface.
Recent investigations into the role of Schwann cells as APCs in M. leprae infection have provided preliminary indication of involvement of this neural cell population in the immunomodulation of neuritic leprosy. Workers also have reported mycobacterial hsp70, one of the immunodominant antigens in M. leprae infection, as an important antigen in the activation of MHC class II-restricted T cells by Schwann cells.42 Coculture of hsp70, antigen-specific CD4 + T cells and Schwann cells result in induction of MHC class II and upregulation of MHC class I expression. This stimulates the production of IFN-7 by antigen-specific CD4+ T cells, causing further upregulation of MHC class II molecules. This response is suggestive of preferential Th 1 subset induction by Schwann cells43 and subsequent macrophage activation which could, in part, explain the correlation of severe nerve damage with tuberculoid as opposed to lepromatous disease.
It is known that Schwann cells also produce endogenous hsp70 in response to stress, such as during nerve injury or pyrexia. Immunocytochemical studies indicate that endogenous hsp70 is produced in peripheral nerves following infection with M. leprae. 44 Importantly, there is significant homology between mycobacterial and mammalian hsp70, represented by a 47% identity at the amino-acid level. Because T cells specific for mycobacterial hsp70 proliferate in response to incubation with human hsp70 pulsed Schwann cells, similar epitopes are recognized within these two proteins, suggesting a potential for crossreactivity. T-cell recognition of human hsp70 previously primed by recognition of mycobacterial hsp70 could lead to an autoimmune reaction directed at stressed nervous tissue, and thus amplify nerve damage in leprosy. This could damage Schwann cells, causing intracellular proteins to be released for presentation by migrating macrophages. Thus, previously sequestered nerve-specific antigens could become targets of immunity leading to the antinuclear and antinerve autoantibodies observed in leprosy.45-46
Reactional states in leprosy
Reactional events in leprosy are centered around episodic deviations from the relatively stable poles of the disease spectrum,47 resulting in reversal of CMI unresponsiveness or inappropriate induction of humoral immunity. These dynamic and often rapid changes in immune reactivity give rise to the tissue damage that is characteristic of the destructive pathology of leprosy. Two of the most frequent immunopathological phenomena in leprosy are erythema nodosum leprosum (ENL) and reversal reactions (RR). In ENL, there is a transitory improvement in CMI in which spontaneous changes in local immunity produce painful erythematous nodules, pyrexia, polyarthralgia, panniculitis and neuritis. The reversal reaction, frequently observed in the early stages of antileprosy therapy, is thought to be a genuine improvement or upgrading of host immunity to M. leprae infection, with enhanced cytocidal activity and transient reduction of the bacillary load. The skin lesions in these patients are distinct from ENL, and usually present as erythematous indurations in regions of skin involved by leprosy lesions. However, similar reactions are also seen in patients whose condition is deteriorating or whose infection has become drug resistent. Although both ENL and RR represent immune activation against M. leprae, they both produce pathological changes in the host, primarily due to immunologically mediated tissue damage.
Abberant immune activation in ENL. The histopathology of ENL is suggestive of an Arthus-type reaction; early nodules contain large numbers of polymorphonuclear leukocytes (PMNL), but in mature lesions lymphocytes predominate. There is marked CD4+ T-cell infiltration, but the tissue damage in affected regions seems to be mediated by local immune complex deposition and complement fixation. The cytokine patterns in ENL are characteristic of Th2 subset activation, with increased expression of IL-6, IL-8, and IL-10 and persistent expression of IL-4 and IL-5 mRNA in affected lesions, detected by PCR amplification of cytokine message.48 Presumably, this T-cell population provides help to B-cell antibody production, resulting in an M. leprae-spe cific humoral response and its attendant pathology. IL-8 is a potent neutrophil chemoattractant that could augment the immediate-type hypersensitivity character of ENL reactions. Additionally, the protective Th1 response may be suppressed by IL-10 production. Recently, complement deficiency has been suggested to contribute to the antibody-mediated pathology in leprosy. A positive association found between the nonexpresscd C4B allele, C4B*Q0, and ENL suggests a role for ineffective complement function in the persistence of immune complexes.49 Thus, reduced immune-complex clearance may lead to the type III hypersensitivity also characteristic of ENL.
Reversal reactions. The reversal reaction (RR) is associated with an improved DTH response to M. leprae antigens (Mitsuda reaction) that is thought to reflect a transient upgrading of protective immunity to infection. As in ENL, there is increased CD4 + T-cell infiltration into lesions, although the cytokine profile of immune activation is more characteristic of a Th1 pattern, in contrast to the Th2 pattern observed in ENL. There is increased IL-1β, TNF-α, IL-2 and IFN-γ production with reciprocally down-regulated IL-4, IL-5 and IL-10 secretion.48
The antigen-specific DTH response is thus driven by a Th1 subset of the CD4+ population. However, little is known of the mycobacterial antigen(s) that mediate this response. Interestingly, the T cells of RR lesions shows a restricted TCR repertoire, with overepresentation of Vβ-12, 14, 19 and particularly Vβ6 elements, suggesting that repertoire selection in this DTH response is driven by a small set of M. leprae antigens.32 Moreover, skewing of the repertoire also seems to be associated with particular HLA restriction elements, with increased V/36 representation in HLA-DR15- and HLADR17-positive individuals (both subspecificities of the HLA-DR3 serotype). This suggests a role for MHC class II in the selection of the T-cell repertoire in reversal reactions, which may account for the differences in Vβ-restriction between HLA-unidentical individuals, and the oligoclonal repertoire observed in the individual.
Immunotherapy of Leprosy; Manipulating the Immune Response Need for a leprosy vaccine
Vaccination is the safest and most cost-effective method of protecting against infectious diseases. Over the time of their employment, vaccines have provided protective immunity to many infectious diseases, including polio, smallpox, tetanus and rubella, having achieved significant success in reducing the incidence of these diseases in the Western hemisphere. However, many of the infectious diseases virtually eradicated from developed countries remain endemic in the developing world, where they make a very significant contribution to rates of morbidity and mortality. Despite intensive research worldwide, an effective vaccine for leprosy, providing consistent and global protection, remains elusive. However, recent advances in recombinant DNA technology and continuing progress in our understanding of the immune system and the mechanisms for the induction of protective immunity, are slowly allowing the design of vaccines that are able to prophylactically fortify protective arms of the immune system against specific infectious organisms. Some of these vaccines are now being tested in large trials in the field, with qualified success.
BCG: a substrate for the development of second generation vaccines? The elucidation of the immunological parameters involved in determining protection and susceptibility in leprosy has provided the theoretical framework which allows a rationalization of vaccine research and development. An improved understanding of the Th1/Th2 dichotomy, and the association of the Th1 response with protective immunity in leprosy, has led researchers to investigate methods of artificially inducing such a response that is safe and specific for M. leprae. The induction of protective CMI is increasingly being recognized as the essential factor in designing an effective vaccine. To this end, the bacille Calmette-Guerin (BCG) has been the most successful in protecting against tuberculosis and, to a significant extent, against leprosy. The hsp65 of M. leprae is an immunodominant antigen in both tuberculosis and leprosy.50 T cells from leprosy patients recognize hsp65 of M. bovis, M. bovis BCG in addition to M. leprae. It is interesting that BCG vaccination for tuberculosis also provides 20%-80% protection from leprosy in India and Africa, suggesting immunological crossreactivity between mycobacterial hsp65 as the mechanism of crossprotection to leprosy by BCG vaccination. However, immunization with BCG has had mixed results in different trials and immunization initiatives, demonstrating a need for a more pathogen-specific, broad-based vaccine with protective CMI-inducing capabilities.
Old bug, new tricks. BCG is a live, attenuated, bovine tubercle bacillus used to immunize against tuberculosis that offers some unique advantages for developing vaccines using recombinant DNA techniques. BCG is the most widely used vaccine in the world, having been administered to 2.5 billion people worldwide with few serious contraindications. Moreover, the immunity developed is long-lived, due to the intrinsic adjuvanticity and heat stability of the bacillus, and is convenient to administer as well as inexpensive to manufacture.
Advances in recombinant DNA technology have led to pioneering new methods for inducing protective immunity to M. leprae by the expression of immunologically relevant mycobacterial antigens in recombinant BCG (rBCG).51 The innocuous persistence of BCG in the host makes it an ideal vector for the induction of long-term protective memory, providing a vehicle for expression of multiple antigenic regions of M. leprae that may be able to preferentially mobilize the Th1 subset, essential for protective cytokine responses, as well as inducing CTL. Investigations into the suitability of rBCG for vaccine development suggest that it is possible to equip BCG, by extrachro- mosomal or integrative mycobacterial transformation, with a multiple cloning site which may be used for the stable expression of M. leprae antigens driven by hsp regulatory elements. This would allow efficient, high copy expression of mycobacterial antigens within the relevant immune compartment for the induction of protective Th1 and CTL responses. This system could also conceivably be used to treat M. leprae infection.
There is much to be learned about the mode of delivery of vaccines and the nature of subsequent immune activations. Evidence suggesting that the route of administration and, consequently, the population of specialized, often tissue-specific, immune cells encountered, has a bearing on the type of immunity evoked, is of importance in devising effective vaccination strategies. The recent attention given to mycobacterial hsp as immunodominant antigens relevant to vaccine development must be treated with caution since immunity to mycobacterial hsp could become misdirected at the cognate host hsp due to their significant homology.
Results from the field. A large study of DTH responses using skin tests with soluble tubercle and leprosy antigens, carried out in northern Malawi, has produced some intriguing relationships between vaccination, DTH responses and protective immunity.52 It revealed that tuberculin positivity, a classical test for assessing antituberculosis protective immunity,53 correlates with protection from leprosy. Further, positivity to second-generation M. leprae- soluble antigens (MLSA) is associated with protection from leprosy, suggesting previous exposure to the leprosy bacillus may contribute to protection. However, BCG vaccination also produces MLSA sensitivity but not protection, possibly due to the differences in the mode of exposure to bacillary antigens in vaccination and infection. The finding that BCG protects against leprosy without persistent skin-test positivity suggests that induction of DTH is not sufficient nor essential in the induction of protective immunity by BCG vaccination. However, it is observed that naturally induced DTH, by exposure to M. leprae and other mycobacteria, strongly correlates with protection. A complete dissection of these findings will require greater knowledge of the mechanisms by which natural and vaccine-induced immunity is generated. The study also calls into question the relevance of DTH assessment as an indication of BCG vaccination efficacy.
Cytokine therapy in leprosy
Elucidation of the cytokines that mediate resistance to M. leprae infection in rodent models, and an indication of similar patterns in man, have led to attempts to induce protective cytokine responses and switch off pathological reactions in leprosy by the use of recombinant cytokines and anticytokine antibodies. The Th1/Th2 paradigm of the rodent model of immunity to infection has been central in identifying suitable candidate cytokines in experimental therapeutic regimens. Successful immunomodulation toward a Th1 cytokine response in lepromatous murine disease, using recombinant Th1 cytokines, has encouraged investigations into the possibility of recombinant cytokine therapy in leprosy.
IL-2 and IFN-γ. Reasoning that the administration of Th1 lymphokines would mimic the protective cytokine milieu observed in resistant individuals, investigators have administered low-dose IL-2 and IFN-γ to lepromatous patients with the intent of reconstituting CMI and reducing the bacillary load. Moreover, evidence from animal models and the study of lymphocyte ontogeny and development suggest that the cytokine environment of naive lymphocytes can determine their subsequent differentiation into Th1/Th2 subsets. Thus, Th1 cytokines were administered in an attempt to induce this subset by simulating the autocrine and paracrine expansion normally undergone in activation.
Low-dose recombinant IL-2 therapy, given as 10 µg intradermal injection every 12 hours for 8 days to lepromatous leprosy patients, produced morphologic changes in the lepromatous lesions.54 This was visualized under the electron microscope as modification of the endothelial surface of arterioles, in the form of blebbing and vacuolation; a marked cellular infiltration into the lesions and changes in their cellular composition. Expression of MHC class II molecules was induced, suggesting production of IFN-γ from Th1 T cells recruited to the lesions. In contrast to lepromatous lesions containing few T cells, that are primarily CD8 + , and large numbers of infected macrophages, IL-2-treated lesions showed extensive recruitment of CD4+ T cells that predominated in the lesions. There was a progressive reduction in bacillary load (a fivefold reduction in mycobacteria during the first 2 months of treatment, 10-20 times that expected with chemotherapy alone) concomitant to IL-2 therapy as well as an enhancement of the humoral response to M. leprae. This antibody response could be secondary to the cytolytic action of CD4 + , CD8+ T cells and macrophages that would result in the release of large amounts of M. leprae- derived antigens for B-cell activation.
Macrophages are seen to be unresponsive and heavily burdened with M. leprae in LL leprosy. Thus, it was reasoned that treatment with IFN-γ, a potent activator of macrophages, would induce a shift toward the tuberculoid pole of disease. Intradermal injection of patients with lepromatous leprosy with 1 to 10 µ g of IFN-γ resulted in a reconstitution of DTH.55 There was a 10-fold decrease of bacilli in half the patients and macrophages/monocytes showed improvement in their cytocidal secretory function.
Adverse effects of lymphokine therapy. Patients undergoing IL-2 therapy develop nontender, axillary lymphadenopathy but are otherwise free of contraindications. Likewise, IFN-7 therapy is tolerated well, with serum antibodies becoming undetectable 28 days after administration. Although cytokine immunomodulation is clearly effective in lepromatous leprosy, the induction of reactional states due to a sudden upgrading of DTH, resulting in acute immunologic and inflammatory reactions, is a potential hazard.56 The fine balance that exists between protective immunity and immunopathology demands very careful and stringent control of the cytokine and cellular cascades in leprosy if cytokine therapy is to become a mainstay of leprosy treatment.
A Unified Immunology of Leprosy; an Eclectic Synthesis Emergent patterns in cellular dynamics of leprosy
Various subsets of the immune-cell compartment have been implicated to play a role in the response to M. leprae infection. The central mediator of specific immunity seems to be the CD4+ T lymphocyte, responsible for the distinct patterns of cytokines and their clinical correlates observed in the disease spectrum. The development of the Th1/Th2 paradigm in animal models of infection and its application to human disease has led to fundamental insights into the dynamic character of the immune response to infection. The macrophage also is prominent as a major player in leprosy, with well-established roles in granuloma formation and cytocidal activity, as well as being the primary reservoir for infection. Recent advances in the parameters that control these functions and their failure in lepromatous disease suggest a vital role for macrophages in the early stages of infection when specific immunity is not maximally activated. The genetic factors regulating the response of macrophages, such as the bcg gene, help to explain the spectrum of mycobacterial burden, from paucibacillary to multibacillary, seen in the disease. The CD4 + Ts cell has been demonstrated to specifically suppress M. leprae -specific Th cells, and this is mediated by an HLA-presented M. leprae epitope of an immunodominant antigen in leprosy infection, mycobacterial hsp65. The later phase of the immune response to M. leprae infection is antigen-specific and is controlled primarily by the HLA class II locus. Thus, specific alleles, particularly of the DR-isotype, confer protection and susceptibility to infection. This effect is most likely mediated by the peptide-binding characteristics of these molecules, with some alleles better able to present key epitopes for protective T-cell responses. The APC also seems to play an important role in determining the type of immunity induced. Macrophages as APCs are able to induce a Th1 protective response; whereas B cells as APCs elicit an immunologically detrimental Th2 response. This suggests that macrophage-specific costimulatory signals might operate in antigen recognition of presented peptide to elicit a Th1 response. Such costimulatory molecules could be expressed by activated macrophages, as is the B7-family of molecules in activated B cells.57 A summary of the cellular dynamics of leprosy is given in Figure 2.
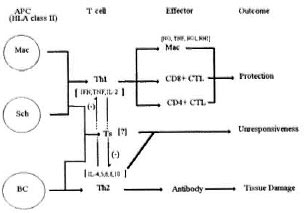
Fig . 2. Schematic of the cellular network regulatingimmunity to M. leprae infection. Mac = macrophage; Sch = Schwann cell; BC = B cell.
A genetic theory of immunoresistance
Genetic studies in murine models reveal that differences in resistance and susceptibility to infection are controlled by the expression of a single dominant gene that exists in two allelic forms, bcgr and bcgs The bcgr allele confers resistance and is dominant over bcgs that represents susceptibility to disease.20 A murine candidate for bcg, Nramp, has recently been described. Although the precise function of Nramp has yet to be determined, it is known that the gene is preferentially expressed in macrophages imparting a differential cytocidal and cytostatic capacity to these cells. Nramp has significant homology with the ATP-binding transporter family of integral membrane proteins as well as being structurally similar to a bacterial nitrite transporter. Interestingly, the Nramp protein is also thought to have a signal-transducing function in macrophage activation. Thus, it would be very likely for genetic polymorphism at this locus to determine, to a significant degree, the immune reaction to infection.
The immunogenetics of leprosy suggests HLA class II molecules to be important in determining the evolution of the immune response against M. leprae infection. Since HLA molecules are codominantly expressed, and considering the extensive allelic polymorphism at this locus, there may be a wide range of immunity to M. leprae antigens in HLA-discordant individuals or between populations with different genetic profiles. Generally, it is believed that HLA-DR isotypes lead to protective responses whereas HLA-DQ produces lepromatous disease. The pivotal role of the HLA system in controlling CMI makes it likely that differences in HLA haplotypes may contribute to the wide spectrum of immunity seen in leprosy (Fig. 3).
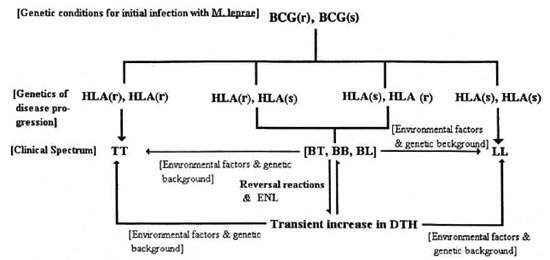
Fig. 3. A multifactorial polygenic model describing the clinical spectrum of disease in leprosy. BCG(r) =resistant beg allele; BCG(s) = susceptible bcg allele; HLA(r) = resistant HLA haplotype; HLA(s) = predisposingHLA haplotype; TT, BT, BB, BL, LL = spectrum of disease activity in leprosy. BCG(r) is assumed to be dominantin the human as an extrapolation of murine genetics and, thus, it is argued that the recessive genotype BCG(s) is the only genotype allowing effective initial infection. The course of disease and, in turn, the clinical outcomeis then determined by the HLA haplotype. The model uses two genetic elements but, of course, there may be many others.
Evidently the genetic determinants of resistance to leprosy cannot be described by classical Mendelian patterns. This complex genetic trait of resistance to M. leprae infection, with at least two contributing loci, is better characterized within the multifactorial polygenic paradigm of the autoimmune diseases. The HLA system as well as other genetic loci are important in the pathogenesis of autoimmune diseases, as are environmental events such as infection and diet. As in leprosy, predisposition to autoimmune disease varies between allelically different individuals, and between different races.
Host-parasite antagonism: a battle for immunological control
Parasites have evolved ways of evading the host's immune system, thus allowing them to persist and replicate in the host, leading to chronic infection. Strategies of immune evasion by intracellular parasites, particularly those of macrophages, have been shown to include downregulation of MHC expression, inhibition of phagosome-lysosome fusion, and shutdown of host cell cytocidal metabolism.58 However, the precise methods by which such parasites evade the host's immune response are only recently becoming apparent.
Workers recently have found evidence for active manipulation of the host's immune system by the parasite. Granuloma formation in schistosomiasis is thought to arise from a classical host DTH response against infection, triggered by egg deposition by Schistosoma mansoni, an helminthic parasite. Host TNF-α has been shown to critically control granuloma formation in schistosomiasis.59 Intriguing new evidence suggests that schistosomes use TNF-α as a reproductory stimulus, increasing their egg output. These exciting new findings have led to speculation that schistosomes manipulate the host cytokine response not only to improve their reproductive capability but also to induce granuloma formation to envelope their eggs, stimulating their exit into the host digestive tract. Moreover, the cytokine profile in the schistosomal granulomas is not characteristic of the protective Th1 pattern expected in DTH, but resembles an aberrant Th2 pattern.
It is interesting to speculate that similar manipulation of the host cytokine response takes place in leprosy leading to the chronic infection characteristic of the disease. As in schistosomal infection, there seems to be an inappropriate cytokine response and granuloma formation, with Th2 cytokine profiles. Indeed, cytokine depletion studies correlate with decreased granuloma formation in infection with a related mycobacteria, M. bovis. The Th2 cytokines could aid mycobacterial survival by various means. Once inside its intracellular niche other mycobacterial factors could ensure their persistence by countering cidal mechanisms. Recent work suggests that M. leprae gain access to the internal environment of the macrophage with the assistance of the complement factors C3 and Clq. Natural antibodies in nonimmune individuals have reactivities for mycobacterial antigens at very low titers. Reactivities to phenolic glycolipid-I (PGLI), a major surface glycolipid of M. leprae, are particularly evident and C3 fixation to this component is strictly antibody dependent.60 Formation of this trimolecular complex allows binding to the complement receptors (CR1, CR3 and CR4) expressed on macrophages, resulting in enhanced internalization of bacilli.61 Once infection is established, such a mechanism may amplify, by positive feedback, due to the induction of a Th2 response with consequent antibody production. The Th2 response also could prevent macrophage activation by down-regulating Th1 T cells via IL-10. Moreover, M. leprae may also express host-dependent virulence genes,62 described in some bacterial infections, which could allow active modulation of host immunity.
Contemplating the future
There have been exciting new developments in the immunology of leprosy, over the past decade, made possible by important advances in our understanding of the immune system and the revolution in molecular biology. However, many fundamental questions remain unanswered about the nature of immunity in leprosy and the perplexing array of immunological phenomenon associated with pathology. The failure, to date, of attempts at culturing M. leprae in vitro restricts the modes of investigation available to leprosy research. However, the development of a mouse foot pad model of M. leprae infection and, more recently, the successful induction of systemic chronic leprosy infection in the armadillo have provided valuable insights into the microbiology of the disease.63 Such models also have been instrumental in the development of antileprosy drugs. An effective vaccine to M. leprae infection is yet to be developed, although increased knowledge of the mechanisms of immune activation and their relevance to the immunoregulation of infection promises rapid progress. The characterization of M.leprae -specific epitopes of mycobacterial antigens, especially the heat-shock proteins, will be useful in the search for a leprosy vaccine and may be suitable for epidemiological and diagnostic applications.
- Kaushik Choudhuri
1. Ridley, D. S. and Jopling, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 31(1966)255-273.
2. Austen, K. F., Talmadge, D. W., Samter, M, Frank, M. M. and Caiman, H. N., eds. Immunological Disease. 4th edn. Boston: Little, Brown & Co., 1988.
3. Rea, T. H., Bevans, L. and Taylor, C. R. The histopathology of the spleen from a patient with lepromatous leprosy. Int. J. Lepr. 48(1980)285-290.
4. Mandell, G. L., Douglas, R. G., Jr., Bennett, J. E., eds. Principles of Infectious Diseases. 3rd edn. Edinburgh: Churchill Livingston, 1990.
5. Grau, G. E. and Modlin, R. L. Immune mechanisms in bacterial and parasitic diseases: protective immunity versus pathology. Curr. Opin. Immunol. 3(1991)480-485.
6. Wilson, R. A. Immunity and immunoregulation in helminth infections. Curr. Opin. Immunol. 5(1993)538-547.
7. Mosmann, T. R. and Moore, K. W. The role of IL-10 in crossregulation of Th1 and Th2 responses. Immunol. Today 12(1991) A49-A53 (59 ref.).
8. Gajewski, T. F., Pinnas, M., Wong, T. and Fitch, F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J. Immunol. 146(1991)1750-1758.
9. Yamamura, M., Uyemura, K., Deans, R. J., Weinberg, K., Rea, T. H., Bloom, B. R. and Modlin, R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277-279.
10. Kaplan, G., Kiessling, R., Teklemariam, S., Hancock, G., Sheftel, G., Job, C. K, Converse, P., Ottenhoff, T. H. M., Becx-Bleumink, M., Dietz, M. and Cohn, Z. A. Reconstruction of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J. Exp. Med. 169(1989)893-907.
11. Reed, S. G. and Scott, P. T-cell and cytokine responses in leishmaniasis. Curr. Opin. Immunol. 5(1993)524-531.
12. Kumar, V., Katoch, K., Katoch, V. M. and Bharadwaj, W. P. A preliminary study of immuno-histological and ultrastructural characteristics of neural granuloma in leprosy patients. Acta Leprol. (Geneve) 8(1992)87-94.
13. Liew, F. Y. and Cox, F. E. G. Nonspecific defence mechanisms: the role of nitric oxide. Immunol. Today 12(1991)A17-A21 (75 ref).
14. Orme, I. M. Immunity to mycobacteria. Curr. Opin. Immunol. 5(1993)497-502.
15. Fong, T. A. and Mosmann, T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J. Immunol. 143(1989)2887-2893.
16. Schurr, E., Morgan, K., Gros, P. and Skamene, E. Genetics of leprosy. Am. J. Trop. Med. Hyg. 44(1991)4-11(55 ref.).
17. Vidal, S. M., Malo, D., Vogan, K., Skamene, E. and Gros, P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Beg. Cell 73(1993)469-485.
18. Unkles, S. E., Hawker, K. L., Grieve, G., Campbell, E. I., Montague, P. and Kinghorn, J. R. crnA encodes a nitrate transporter in Aspergillus nidulans. Proc. Natl. Acad. Sci. U.S.A. 88(1991)204-208.
19. Formica, S., Roach, T. I. and Blackwell, J. M. Interaction with extracellular matrix proteins influences Lsh/Ity/Bcg (candidate Nramp) gene regulation of macrophage priming/activation for tumour necrosis factor-α and nitrite release. Immunology 82 (1994) 42-50.
20. Gros, P., Skamcne, E. and Forget, A. Genetic control of natural resistance to Micobacterium bovis (BCG) in mice. J. Immunol. 127(1981)2417-2421.
21. Hormaeche, C. E., Harrington, K. A. and Joysey, H. S. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J. Infect. Dis. 152(1985)1050-1056.
22. Brett, S., Orrell, J. M., Beck, J. S. and Ivanyi, J. Influence of H-2 genes on growth of Mycobacterium tuberculosis in the lungs of chronically infected mice. mmunology 76(1992)126-132.
23. Germain, R. N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell 76(1994)287-299(81 ref.).
24. Falk, K., Rotzsche, O., Stevanovic, S., Jung, G. and Ramensee, H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351(1991)290-296.
25. Engelhard, V. H. Structure of peptides associated with MHC class I molecules. Curr. Opin. Immunol. 6(1994)13-23(110 ref.).
26. Rotzschke, O. and Falk, K. Origin, structure and motifs of naturally processed MHC class II ligands. Curr. Opin. Immunol. 6(1994)45-51.
27. de Vries, R. R. P. and Ottenhoff, T. H. M. Immunogenetics of leprosy. In: Leprosy. 2nd edn. Hastings, R. C, ed. Edinburgh, Churchill Livingstone, 1994, 113-121.
28. Aizawa, M. DRw53. In: HLA in Asia-Oceania. Aizawa, M., ed. Sapporo: Hokkaido University Press, 1986, p. 175.
29. Hill, A. V., Elvin, J., Willis, A. C, Aidoo, M., Allsopp, C. E., Gotch, F. M., Gao, V. M., Takiguchi, M., Greenwood, B. M., Townsend, A. R., et at. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360(1992)434-439.
30. The single leter amino-acid code is translated as follows: A = alanine; C = cysteine; D = aspartate; E = glutamate; F = phenylalanine; G = glycine; H = histidine; I = isoleucine, K = lysine, L = leucine; M = methionine; N = asparagine; P = proline; Q = glutamine; R = arginine; S = serine; T = threonine; V = valine; W = tryptophan; Y = tyrosine.
31. Geluk, A., Van Meijgaarden, K. E., Janson, A. A., Drijfhout, J. W., Meloen, R. H., de Vries, R. R. P. and Ottenhoff, T. H. M. Functional analysis of DR17 (DR3)-restricted mycobacterial T-cell epitopes reveals DR17-binding motif and enables the design of allele-specific competitor peptides. J. Immunol. 149(1992)2864-2871.
32. Wang, X., Golkar, L., Uyemura, K., Ohmen, J., Villahermosa, L. G., Fajardo, T. T. Jr., Celona, R. V., Walsh, G. P. and Modlin, R. L. T cells bearing Vβ6 T cell receptors in the cell-mediated immune response to Mycobacterium leprae. J. Immunol. 151(1993)7105-7116.
33. Elson, C. J. and Thompson, S. J. Immunity, autoimmunity and immunotherapy: new frontiers in heat shock protein research. (Editorial review) Clin. Exp. Immunol. 98(1994)175-177.
34. Adams, E., Garsia, R. J., Hellqvist, L., Holt, P. and Basten, A. T cell reactivity to the purified mycobacterial antigens p65 and p70 in leprosy patients and their household contacts. Clin. Exp. Immunol. 80(1990)206-212.
35. Adams, E., Britton, W. J., Morgan, A., Godsall, A. L. and Basten, A. Identification of human T cell epitopes in the Mycobacterium leprae heat shock protein 70 kD antigen. Clin. Exp. Immunol. 94(1993)500-506.
36. Mehra, V., Bloom, B. R., Bajardi, A. C, Grisso, C. L., Siding, P. A., Alland, D., Convit, J., Fan, X.,Hunter, S. W., Brennan, P. J., Rea, T. H. and Modlin,R. L. A major T cell antigen of Mycobacterium leprae is a 10 kD heat-shock cognate protein. J. Exp. Med. 175(1992)275-284.
37. Ottenhoff, T. H. M., Elferink, D. G., Klatser, P. R. and de Vries, R. R. P. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature 332(1986)462-464.
38. Haregewoin, A., Godal, T., Mustafa, A. S., Belehu, A. and Yamaneberhan, T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
39. Mutis, T., Cornelisse, Y. E., Datema, G., van den Elsen, P. J., Ottenhoff, T. H. M. and de Vries, R. R. P. Definition of a human suppressor T-cell epitope. Proc. Natl. Acad. Sci. U.S.A. 91(1994)9456-9460.
40. Sette, A., Sidney, J., Oseroff, C, del Guerico, M. F., Southwood, S., Arrenhius, T., Powell, M. F., Colon, S. M., Gaeta, F. C. and Grey, H. M. HLA DR4w4-binding motifs illustrate the biochemical basis of degeneracy and specificity in peptide-DR interactions. J. Immunol. 151(1993)3163-3170.
41. Mustafa, A. S., Lundin, K. E. A. and Oftung, F. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLA-Dr molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61(1993)5294-5301.
42. Ford, A., Britton, W. J. and Armati, P. J. Schwann cells are able to present exogenous mycobacterial hsp70 to antigen-specific T lymphocytes. J. Neuroimmunol. 43(1993)151-159.
43. Perhaps by differential expression of costimulatory molecules, similar to the B7 family of molecules expressed on activated B cells, that costimulate T cells via CD28 and CTLA-4.
44. Khanolkar-Young, S., Young, D. B., Colston, M. J., Stanley, J. N. A. and Lockwood, D. N. J. Nerve and skin damage in leprosy is associated with intralesional heat shock protein. Clin. Exp. Immunol. 96(1994)208-213.
45. Park, J. Y., Cho, S. N., Youn, J. K., Kim, D. I., Cellona, R. V., Fajardo, T. T., Jr., Walsh, G. P. and Kim, J. D. Detection of antibodies to human nerve antigens in sera from leprosy patients by ELISA. Clin. Exp. Immunol. 87(1992)368-372.
46. Garcia-de Torre, I. Autoimmune phenomena in leprosy, particularly antinuclear antibodies and rheumatoid factor. J. Rheumatol. 20(1993)900-903.
47. Patients who experience reversal reactions usually have lepromatous disease and progress toward the tuberculoid pole of immune responsiveness.
48. Yamamura, M., Wang, X.-H., Ohmen, J. D., Uyemura, K, Rea, T. H., Bloom, B. R. and Modlin, R. L. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149(1992)1470-1475.
49. de Messias, I. J. T., Santamaria, J., Brenden, M., Reis, A. and Mauff, G. Association of C4B deficiency (C4B*Q0) with erythema nodosum leprosum in leprosy. Clin. Exp. Immunol. 92(1993)284-287.
50. Husson, R. N. and Young, R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc. Natl. Acad. Sci. U.S.A. 84(1987)1679-1681.
51. Stover, C. K., de la Cruz, V. F., Fuerst, T. R., Burlein, J. E., Benson, L. A., Bonnette, L. T., Bansal, G. P., Young, J. F., Lee, M. H., Hatfull, G. F., Snapper, S. B., Barletta, R. G., Jacobs, W. R., Jr. and Bloom, B. R. New use of BCG for recombinant vaccines. Nature 351(1991)456-460.
52. Fine, P. E. M., Sterne, J. A. C, Ponnighaus, J. M. and Rees, R. J. W. Delayed-type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet 344(1994)1245-1249.
53. This DTH is not completely protective and is responsible for the substantial immunologically mediated tissue damage of reversal reactions.
54. Kaplan, G., Britton, W. J., Hancock, G. E., Theuvenet, W. J., Smith, K. A., Job, C. K, Roche, P. W., Molloy, A., Burkhardt, R., Baker, J., Pradhan, H. M. and Cohn, Z. A. The systemic influence of recombinant interleukin 2 on the manifestation of lepromatous leprosy. J. Exp. Med. 173(1991)993-1006.
55. Nathan, C. F., Kaplan, G., Levis, W. R., Nusrat, A., Witmer, M. D., Sherwin, S. A., Job, C. K., Horowitz, C. R., Steinman, R. M. and Cohn, Z. A. Local and systemic effects of intradermal recombinant interferon-γ in patients with lepromatous leprosy. N. Engl. J. Med. 315(1986)6-15.
56. Sampaio, E. P., Moreira, A. L., Sarno, E. N., Malta, A. M. and Kaplan, G. Prolonged treatment with recombinant interferon-γ induces erythema nodosum leprosum in lepromatous leprosy patients. J. Exp. Med. 175(1992)1729-1737.
57. Galvin, F., Freeman, G. J., Razi-Wolf, Z., Hall, W., Jr., Benacerraf, B., Nadler, L. and Reiser, H. Murine B7 antigen provides a sufficient costimulatory signal for antigen-specific and MHC-restricted T cell activation. J. Immunol. 149(1992)3802-3808.
58. Hall, B. F. and Joiner, K. A. Strategies of obligate intracellular parasites for evading host defences. Immunol. Today 12(1991)A22-A27 (86 ref.).
59. Amiri, P., Locksley, R. M., Parslow, T. G., Sadick, M., Rector, E., Ritter, D. and McKerrow, J. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356(1992)604-607.
60. Schlesinger, L. S. and Horwitz, M. A. A role for natural antibody in the pathogenesis of leprosy: antibody in the nonimmune serum mediates C3 fixation to the Mycobacterium leprae surface and hence phagocytosis by human mononuclear phagocytes. Infect. Immun. 62(1994)280-289.
61. Schlesinger, L. S. and Horwitz, M. A. Phagocytosis of Mycobacterium leprae by human monocyte derived macrophages is mediated by complement receptors CR1(CD35), CR3(CD11b/CD 18), and CR4(CD11/ CD 18) and IFN-gamma activation inhibits complement receptor function and phagocytosis of this bacterium. J. Immunol. 147(1991)1983-1994.
62. Mahan, M. J., Slauch, J. M. and Mekalanos, J. J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259(1993)686-688.
63. Rees, R. J. W. Evolution and contribution of animal models in leprosy. Indian J. Lepr. 63(1991)446-456.
* This review, written by Kaushik Choudhuri while a third-year student at King's College School of Medicine and Dentistry at the University of London, was the 1994 prize-winning essay in the annual competition sponsored by the British Leprosy Relief Association (LEPRA) for essays on various aspects of leprosy. The author's address is: Kaushik Choudhuri, 20 Cato Road, London SW4 7TX, U.K.