- Volume 63 , Number 3
- Page: 369–80
Analysis of T-cell and B-cell responses to recombinant M. leprae antigens in leprosy patients and in healthy contacts: significant t-cell responses to antigens in M. leprae nonresponders
ABSTRACT
The recognition of a panel of recombinant Mycobacterium leprae antigens by T cells and B cells f rom 29 borderline tuberculoid/ tuberculoid (BT/TT) and 18 lepromatous leprosy (LL) patients and f rom 21 healthy controls (HC) in leprosy-endemic regions of Ethiopia was examined. All 11 antigenic molecules tested (including M. leprae hsp 10, hspl8, hsp65 and several novel M. leprae antigens) were shown to be recognized by T cells, but clear quantitative differences existed between reactivities induced by individual antigens. Similar quantitative differences were observed when antibody responses to hsp 10 and hsp65 antigens were determined. No associations were found between the antigen-specific responses and the subject status of either BT/TT and LL patients or HC. Fifteen percent of the patients who were nonresponsive to sonicates of M. leprae showed significant T-cell responses to one or more individual M. leprae antigens. This indicates that M. leprae constituents other than the proteins tested are responsible for the M. leprae-specific nonresponsiveness in these patients, which may be exploited for the design of vaccines or immunotherapeutic modalities aimed at inducing M. leprae-specific immunity in non-responders.RÉSUMÉ
La reconnaissance d'un panel d'antigénes recombinants de Mycobacterium leprae par des cellules T et B provenent de 29 patients lépreux borderline tuberculoides et tuberculoides (BT/TT), de 18 patients lépromateux (LL) et de 21 témoins en bonne santé de régions d'Ethiopie endémiques pour la lépre a été examinée. On a montré que toutes les 11 molécules antigéniques testées (y compris hsp10, hspl8, hsp65 et plusieurs antigénes "nouveaux" de M. leprae) étaient reconnues par les cellules T, mais que des différences quantitatives évidentes existaient dans la réactivité induite par les antigénes individuels. De semblables différences quantitatives ont été observées quand on a déterminé les réponses en anticorps aux antigénes hsplO et hsp65. Aucune association n'a été trouvée entre les réponses spécifiques pour l'antigéne et le statut individuel des personnes, que ce soient des patients BT/TT ou LL ou des individuels en bonne santé. Quinze pourcents des personnes qui ne répondaient pas aux sonicats de M. leprae montraient des réponses significatives de leurs cellules T à un ou plusieurs antigénes individuels de M. leprae. Ceci indique que des constituants de M. leprae autres que les protéines testées sont responsables de la non-réponse spécifique pour M. leprae chez ces patients, ce qui pourrait être exploité pour la conception de vaccins ou de moyens immunothérapeutiques ayant pour but d'induire une immunité spécifique pour M. leprae chez les non-répondants.RESUMEN
Se examinó el reconocimiento de un panel de antígenos recombinantes de Mycobacterium leprae por las células T y B de 29 pacientes con lepra tuberculoide/ tuberculoide subpolar (BT/TT), de 18 pacientes con lepra lepromatosa (LL), y de 21 controles sanos (HC) de regiones con lepra endémica en Etiopia. Las 11 moléculas antigénicas probadas (incluyendo hsplO, hspl8, hsp65 y varios antígenos nuevos de M. leprae) fueron reconocidos por las células T, aunque existieron claras diferencias cuantitativas entre las reactividades inducidas por los antígenos individuales. También se observaron diferencias cuantitativas similares en el caso de las respuestas en anticuerpo con los antígenos hsp 10 y hsp65. No se encontraron asociaciones entre las respuestas específicas para un antígeno y el estado BT/ TT, LL, o HC de los individuos. Quince porcicnto de los pacientes que no respondieron a sonicados totales de M. leprae mostraron significantes respuestas celulares (Le T) hacia uno o más antígenos individuales de M. leprae. Se concluye que no son las proteínas probadas en este estudio, sino otros constituyents de M. leprae, los responsables de la anergia específica que muestran los pacientes hacia el bacilo de la lepra. Esto podría ser explotado en el diseño de vacunas o de modalidades inmunoterapéuticas tendientes a inducir inmunidad específica hacia M. leprae en los individuos no respondedores.It is believed that upon infection with Mycobacterium leprae the nature of the T-cell immune response in the exposed individual determines whether leprosy will develop or whether protection will be induced. Healthy exposed individuals and tuberculoid leprosy patients are both characterized by a strong T-cell response to M. leprae which limits infection in healthy individuals but which may be pathogenic to the host in tuberculoid patients. Lepromatous leprosy is typically characterized by a specific T-cell nonresponsiveness to M. leprae and is associated with dissemination of the leprosy bacillus throughout the host.
To identify the molecules of M. leprae involved in these immune responses, biochemical purification procedures and screening of recombinant DNA expression libraries with monoclonal antibodies and patient sera have led to the identification of a large number of protein antigens (reviewed in 33). Analyses of T-cell responses of subjects exposed to M. leprae thus far have indicated that heat-shock proteins (hsp) of 10 kDa, 18 kDa, 65 kDa and 70 kDa, as well as a 36-kDa protein and a family of 30/31-kDa secreted fibronectin-binding proteins are important T-cell antigens (2,6,8,10.11,15-18) However, the contributions of the various antigen-specific T-cell responses to protection or pathogenesis of leprosy remain unclear.
The present study was initiated to examine the ability of T cells as well as B cells to respond to a panel of M. leprae protein antigens in patients and healthy controls in leprosy-endemic regions of Ethiopia. The results demonstrate that distinct differences do exist between the abilities of individual antigens to stimulate proliferation (and antibody production) in the subjects studied. However, these antigen-specific responses did not show a clear association with leprosy status. Thus, no evidence was found for a potential role of particular antigens in the induction of protection or in the pathogenesis of leprosy. Secondly, it was established that the specific nonresponsiveness to M. leprae sonicates in lepromatous (LL) leprosy patients is not reflected by specific nonresponsiveness to any of the M. leprae antigens tested.
MATERIALS AND METHODS
Patients and contacts. Forty-seven leprosy patients, classified according to Ridley-Jopling criteria, were included in this study. Thirty-five patients were recruited from Addis Ababa and the surrounding Ethiopian highlands by the ALERT Leprosy Control Unit. Twelve patients were recruited from the Rift Valley near Sashamene by the National Leprosy Control Programme which is funded by the German Leprosy Relief Association (GLRA). Patients were grouped as paucibacillary [borderline tuberculoid leprosy (BT) = 26; polar tuberculoid leprosy (TT) = 3] and as multibacillary [lepromatous leprosy (LL) = 18]. Forty-two of the patients studied were receiving multiple drug treatment (MDT); five BT patients (BT20, 38, 63, 66, 70) and one TT patient (TT53) were studied before treatment. Twenty-one healthy household contacts (HC) of leprosy patients living in a rehabilitation village near the ALERT hospital in Addis Ababa were studied: 18 contacts were Mitsuda positive, 1 was negative and 2 were not tested. The household contacts were not related to any of the patients involved in this study.
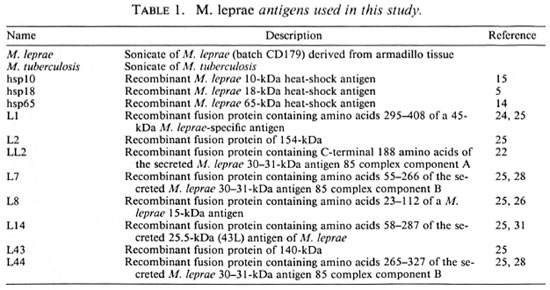
Antigens. The antigens used in this study are listed in Table 1. Purified recombinant proteins M. leprae hsp 10 and hsp65 were obtained from J. van Embden through the WHO/TDR/IMMLEP Special Programme. M. leprae sonicates were obtained from R. Rees; M. tuberculosis sonicates from A. Kolk; purified M. leprae hsp 18 from J. Watson. Escherichia coli strains carrying pEX2 containing M. leprae DNA inserts derived from λgtl 1 recombinants LI, L2, LL2, L7, L8, L14, L43, and L44 (21,24) were established as described (28). Scmipurified crobcta-galactosidase fusion proteins were prepared from induced lysates as described (27). Fusion proteins were approximately 50% pure, as estimated by the protein profiles from SDS/PAGE gels stained with Coomassie brilliant blue.
Peripheral blood mononuclear cells (PBMC) and sera. PBMC were collected by venipuncture using heparinized vacutainer tubes (Becton, Dickinson, Mechelen, Belgium). PBMC were isolated by Ficoll-Paque (Pharmacia, Uppsala, Sweden) centrifugation and resuspended in culture medium containing RPMI-1640 medium (Sigma Chemical Company, St. Louis, Missouri, U.S.A.) supplemented with penicillin (100 IU/mlIBCO, Gaithersburg, Maryland, U.S.A.) and glutamine (2 mM; ICN Flow, High Wycombe, U.K.). Serum was separated from whole blood and stored, without preservative, at - 70ºC until use.
Proliferation assay. Isolated PBMC (105cells/well) were cultured in 96-well, round-bottom, microtiter plates (Costar Corporation, Cambridge, Massachusetts, U.S.A.) in culture medium containing 10% pooled human serum and antigen. Sonicates of M. leprae and M. tuberculosis were used at two different concentrations of 1 µ g/ml and 10 µ g/ml; purified recombinant M. leprae antigens at concentrations of 2 µ g/ml and 20 µ g/ml. Semipurified fusion proteins were used at dilutions of 1/100 and 1/5000. Control wells contained either phytohemagglutinin (PHA, 2 µ g/ml; Wellcome Diagnostics, Dartford, U.K.), semipurified crobetagalactosidase (nonfused) proteins, or culture medium alone. Cultures were set up in triplicate and incubated for 6 days at 37ºC in a fully humidified atmosphere containing 5% C02 . For the last 18 hr, the cultures were pulsed with 1.0 µ Ci of 3 H-thymidine (specific activity, 5.0 µ Ci/mmol; Amersham International, Amersham, U.K.) per well. The cells were then harvested onto glass-fiber filters with a semi-automatic sample harvester. 3H-thymidine incorporation was assessed by liquid scintillation spectroscopy. The results of the responses to sonicates of M. leprae, M. tuberculosis and to the purified proteins hsp10, hsp18 and hsp65 are expressed as a stimulation index (SI) which is the ratio of 3H-thymidine incorporation of antigen-stimulated cultures to that of the control cultures, containing neither antigen nor PHA. The results of the responses to the (semi)purified recombinant fusion proteins LI, L2, LL2, L7, L8, LI4, L43 and L44 are expressed as SI, which is the ratio 3H-thymidine incorporation of antigen-stimulated cultures to that of cultures containing semipurified crobeta-galactosidase proteins as expressed by pEX2 vector. A SI of > 3 was taken as a positive response. The average PHA responses were 26, 805 counts per minute (cpm) for the 21 HC, 25,031 cpm for the 29 BT/TT patients, and 19, 453 cpm for the 18 LL patients.
Immunoblotting and ELISA. Eight percent sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (9). Either 200 ng purified hsp10 or 50 ng purified hsp65 were loaded per lane. Gels were blotted onto nitrocellulose membranes (BA-83; Schleicher & Schuell, Dassel, Germany) using a 0.2 amp current for 2 hr in blotting buffer according to Maniatis (13). The nitrocellulose membranes were blocked overnight with 50 mM Tris-HCl pH 8.0; 150 mM NaCl; 5% ovalbumin; 0.01 % NaN3 at room temperature prior to incubation with anti-sera. Sera were tested at 1/100 (HC and BT/ TT) or 1/500 (LL) dilutions.
ELISA was performed essentially as described previously (12). Sonicates of M. leprae and M. tuberculosis and hsp10 and hsp65 antigens were tested at 200 ng/well; and phenolic glycolipid-I (PGL-I) coupled to human serum albumin was tested at 50 ng/ well and compared to uncoupled human serum albumin (50 ng/well). Sera were tested at 1/100 dilutions for antibodies reactive with hsp10 and hsp65, and at dilutions of 1/150, 1/400 and 1/600 for sonicates of M. leprae and M. tuberculosis. The serum samples were added to quadruplicate wells of the microtiter plate (Dynatech Immulon): two with antigen and two without antigen. The plates were incubated at room temperature for 2 hr. Alkaline phosphatase conjugated F(ab')2 fragments of affinity isolated anti-human gamma or anti-human mu chain or anti-human gamma, alpha and mu chain (Sigma) were used as secondary antibodies. P-nitrophenylphosphate disodium (Sigma 104 phosphatase substrate tablets) was used as a substrate and the reaction was stopped by the addition of 4 N NaOH after 20 min. Absorbances were read at 405 nm using a Titertek Multiscan spectrophotometer (Flow Labs, Richmond, Virginia, U.S.A.), and mean absorbance values were calculated. The antibody reactivity to the coated antigen for each serum sample was calculated by subtracting the mean absorbance of duplicate samples in buffer-coated wells from the mean absorbance of duplicate antigen-coated wells. Delta absorbances of > 0.20 were considered positive.
RESULTS
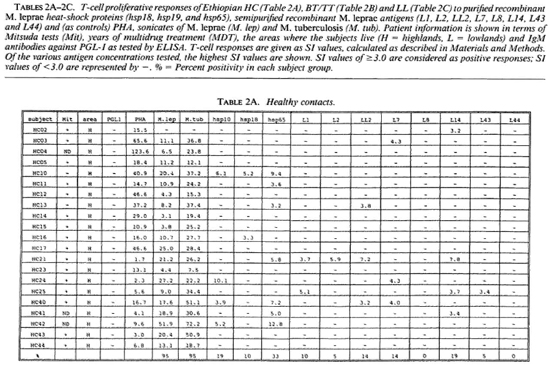
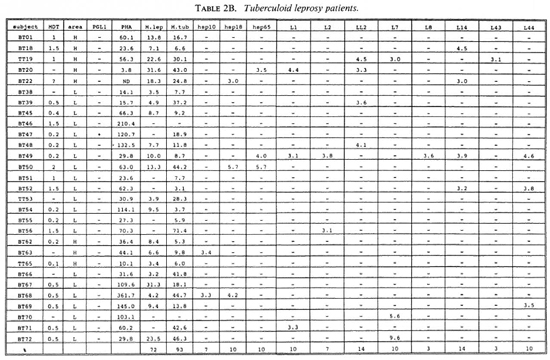
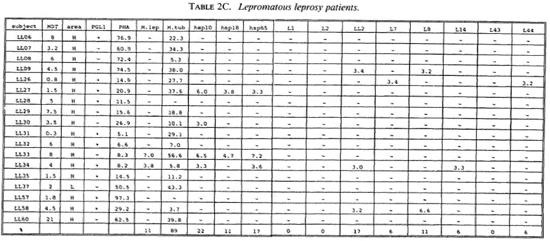
Proliferative T-cell responses to M. leprae antigens. Pilot experiments demonstrated that freshly isolated human peripheral mononuclear cells (PBMC) gave significantly higher T-cell proliferative responses than did frozen PBMC upon stimulation with M. leprae and control antigens (data not shown). Therefore, we performed our studies on fresh PBMC within 48 hr after collection of blood samples. Tables 2A-2C summarize the T-cell proliferative responses of the subjects studied, grouped according to their disease status (HC, BT/TT and LL). Three purified M, leprae antigens (hsp10, hspl8 and hsp65) and eight semi-purified M. leprae proteins fused to E. coli crobeta-galactosidase were tested.
High frequencies of responses to M. leprae sonicates were found in HC (95%) and in BT/TT subjects (72%). Only 11% of the LI patients studied responded to M. leprae sonicate, a finding in agreement with the previously reported M. leprae- specific non-responsiveness at the lepromatous pole of the leprosy spectrum. In all three subject groups the frequency of responses to M. tuberculosis sonicates was high (90%).
A large variability in responses to the three purified and eight semipurified recombinant M. leprae proteins tested was found in the three subject groups. For example, BT patient 49 recognized 6 of 11 T-cell antigens versus BT patient 45 who recognized 0 of 11 T-cell antigens.
Among the purified M. leprae antigens, hsp65 was most frequently recognized in HC (33%), followed by hsp10 (19%) and hsp18 (10%). In patients the frequencies of proliferative T-cell responses to these three antigens were more similar (7%-10% in BT/ TT patients; 11%-22% in LL patients). Among the semipurified M. leprae antigens the frequencies of T-cell responses were highest to antigens LI4 (6%-19%), LL2 (14%-17%) and L7 (6%-14%), all of which are secreted. Frequencies of recognition to the other, nonsecrcted antigens ranged from 0% to 11%. None of the antigens was shown to be exclusively recognized by any of the three subject groups, which would suggest that none of these antigens played a particular role in protection or in disease.
With respect to MDT treatment, no correlation could be demonstrated between duration of treatment and recognition profiles of any tested recombinant M. leprae antigen. No differences in T-cell responses to any M. leprae antigens could be detected between MDT untreated and treated individuals.
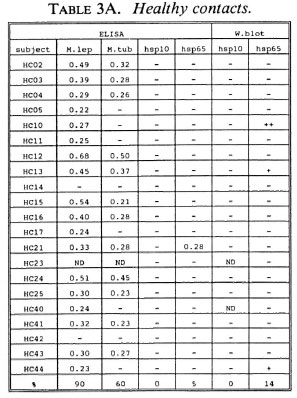
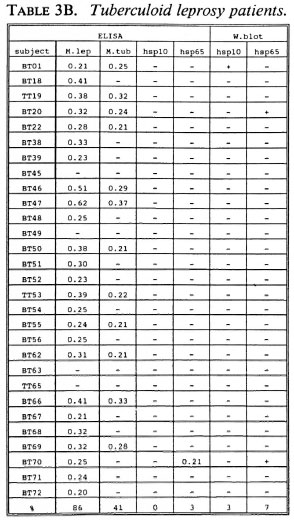
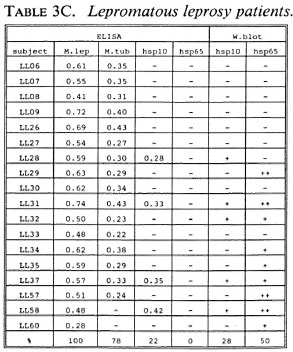
B-cell recognition of M. leprae antigens. To evaluate the antibody responses to purified hsp10 and hsp65 antigens, we employed ELISA and Western blotting techniques (Tables 3A-3C). All LL patients (100%) and the majority of BT/TT patients (86%) and HC (90%) reacted to M. leprae sonicates in ELISA. With respect to M. tuberculosis, serum antibodies were detected in 78% of LL patients, 41% of BT/TT patients and 60% of HC.
Combining the ELISA and Western blotting results, antibodies to M. leprae hsp10 and hsp65 appeared more frequently in the LL group than in the BT/TT and the HC groups. The fact that in the LL group antibodies to hsp65 were only detected by Western blotting and not by ELISA suggests that these antibodies are mainly directed to linear determinants. The existence of mainly linear determinants on hsp65 has been reported previously, and is thought to reflect its predominant existence as an unfolded molecule which is more amenable to proteolytic degradation (34). Comparisons of T-cell versus B-cell responses in the three groups of subjects studied did not reveal any striking correlations (Tables 2A-2C versus3A-3C).
DISCUSSION
In this study we have compared immune responses to 11 M. leprae antigens in 21 healthy household contacts and 47 leprosy patients from Ethiopia, a country where leprosy is endemic.
A major question we wanted to address by this study was whether there is a role for any particular M. leprae antigen in either immunopathology or protection as judged by T-cell proliferation. A role for secreted antigens in protective immunity has been proposed since these antigens are actively secreted by live bacteria and, thus, are available for immune recognition early in infection.
A limited amount of protection in an animal model for tuberculosis after immunization with secreted proteins was recently reported (20). In the present study, we found that the response to secreted antigens, i.e., 30731-kDa and 25.5-kDa antigens, is not restricted to any particular patient group and, thus, is not predictive for protection or pathogenesis. Our study confirms earlier data (3,4,11) that secreted antigens frequently induce proliferative responses (23,31). However, the responses toward heat-shock proteins (hsp) were found to be equally high, and this finding indicates that antigens from different compartments serve as frequent targets for proliferative T cells. Mycobacterial hsp have been implicated in immunopathological phenomena (21). Autologous hsp may form a target for T cells that were originally raised to structurally similar M. leprae hsp molecules. Proliferative T cells specific for mycobacterial hsp65 have been shown to induce autoimmune phenomena in a rat model (30), and it has been hypothesized that a similar reactivity may be involved in some of the immunopathological phenomena associated with leprosy (11). Although a considerable proportion of the subjects in our study recognized hsp65 and other hsp, no support for a particular role for hsp in the induction of immunopathology was found, and hsp were also frequently recognized by healthy individuals.
Thus, with regard to hsp and secreted antigens and the other antigens studied, we found an overall similarity in the antigenic repertoire recognized in healthy individuals and patients. Although a different picture may emerge when using different read-out systems, like cytotoxicity or cytokine production, this similarity in antigen recognition may indicate that the key to distinguishing protective from disease-associated immunity may lie in the exact nature of the T-cell response. It may be that the magnitude, quality and, perhaps, timing of the T-cell response rather than the recognized antigenic repertoire ultimately determines the outcome of an infection with M. leprae.
A potentially important finding in this study was that T-cell nonresponsiveness of 5 LL patients (LL09, 26, 27, 30, 58), 4 BT patients (BT52, 56, 70, 71) and 1 HC (HC02) to M. leprae sonicates is not reflected by nonresponsiveness to individual antigens of M. leprae (Tables 2B-2C). For example, the M. leprae nonresponder LL27 showed significant T-cell proliferation to all three purified M. leprae hsp and the non-responders LL09, LL26, LL58 and BT52 responded to two semipurified crobeta-galactosidase M. leprae fusion proteins. The above finding confirms previous work for recombinant M. leprae antigens (16,18,26), and opens up possibilities to study the mechanisms underlying specific nonresponsiveness to whole M. leprae . The outcome of such studies may, ultimately, be helpful in the design of immunotherapy aimed at triggering antigen-specific immunity in nonresponsive leprosy patients.
Acknowledgment. The authors are grateful to the Ministry of Health, Ethiopia, for permission to perform this study. Sister Genet Amare is gratefully acknowledged for assistance in collecting HC from the leprosy village around the Armauer Hansen Research Institute (AHRI). Dr. Nega Wolde Hawariat of the German Leprosy Relief Association (GLRA) is acknowledged for assistance in collecting BT/TT patients from the Sashcmane area. We express our gratitude to all collaborators of the Leprosy Control Division of the All-Africa Leprosy Training & Rehabilitation Centre (ALC-ALERT) for arranging blood collections in and around Addis Ababa. We wish to thank C. Abou-Zeid, J. van Embdcn, A. Kolk, J. Kamcrbcck, R. Recs, and J. Watson for the supply of antigens. We greatly acknowledge the help of H. Frankcn and A. Boclcn in statistical analysis and T. Ottcnhoff and T. Mutis for critical reading of the manuscript. Finally, we arc highly indebted to Lisette Bontenbal (Sister Clivia) for arranging infinite caloric and culinaric supports during curfew timed experiments. The Armauer Hansen Research Institute (AHRI) is associated with the All-Africa Leprosy Rehabilitation & Training Centre (ALERT) and is supported by the Norwegian and Swedish agencies for international development (NORAD and SIDA). This study also received support from the Dutch Leprosy Relief Association (NSL), the Royal Netherlands Academy of Arts and Sciences (KNAW), and the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR).
REFERENCES
1. Abou-Zeid, C, Smith, I., Grange, J. M., Ratliff, T. L., Steele, J. and Rook, G. A. W. The secreted antigens of M. tuberculosis and their relationship to those recognised by the available antibodies. J. Gen. Microbiol. 134(1988)531-538.
2. A dams, E., Garsia, R. J., Hellqvist, L., Holt, P. and Basten, A. T-cell reactivity to the purified mycobacterial antigens p65 and p70 in leprosy patients and their household contacts. Clin. Exp. Immun. 80(1990)206-212.
3. A nderson, P., Askgaard, D., Ljungqvist, L., Bentzon, M. W. and Heron, I. T-cell proliferative response to antigens secreted from M. tuberculosis. Infect. Immun. 59(1991) 1558-1563.
4. Anderson, P. and Heron, I. Specificity of protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 61(1993)844-851.
5. Booth, R. J., H arris, D. P., L ove, J. M. and Watson, J. D. Antigenic proteins of Mycobacterium leprae; complete sequence of the gene for the 18-kDa protein. J. Immunol. 140(1988)597-601.
6. Dockrell, H. M., Stoker, N. G., Lee, S. P., Jackson, M., Grant, K. A., Jouy, N. F., L ucas, S. B., Hasan, R., Hussain, R. and McAdam, K. P. W. J. T-cell recognition of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1979-1983.
7. Garbe, T., Harris, D., Vordermeier, M., Lathigra, R., Ivanyi, J. and Young, D. Expression of the Mycobacterium tuberculosis 19-kilodalton antigen in Mycobacterium smegmatis: immunological analysis and evidence of glycosylation. Infect. Immun. 61(1993)260-267.
8. K aufmann, S. H. E., Vath, U, Thole, J. E. R., van Embden, J. D. A. and Emmrich, F. Enumeration of T-cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant 64-kDa protein. Eur. J. Immunol. 17(1987)351-357.
9. Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 226(1970)680-685.
10. Launois, P., Huygen, K., DeBruyn, J., N'Diaye, M., Diouf, B., Sarthou, J.-L., Grimaud, J. and Millan, J. T-cell responses to purified filtrate antigens 85 from Mycobacterium bovis bacilli Calmette-Guerin (BCG) in leprosy patients. Clin. Exp. Immunol. 86(1991)286-290.
11. Launois, P., N'Diaye Niang, M. B., Sarthou, J.-L., Rivier, F., Drowart, A., Van Vooren,J.-P., Millan, J. and Huygen, K. T-cell stimulation with purified mycobacterial antigens in patients and healthy subjects infected with Mycobacterium leprae: secreted antigen 85 is another immunodominant antigen. Scand. J. Immunol. 38(1993)167-176.
12. Lyons, N. F., Shannon, E. J., Ellis, B. P. B. and Naafs, B. Association of IgG and IgM antibodies to phenolic glycolipid-1 antigen of Mycobacterium leprae with disease parameters in multibacillary leprosy patients. Lepr. Rev. 59(1988)45-52.
13. Maniatis, T., Fritsch, E. F. and Sambrook, J. Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 1989.
14. Mehra, V., Sweetser, D. and Young, R. A. Efficient mapping of protein antigenic determinants. Proc. Natl. Acad. Sei. U.S.A. 83(1986)7013- 7017.
15. Mehra, V., Bloom, B. R., Barjani, A. C, Grisso, C. L., Sieling, P. A., Alland, D., Convit, J., Fan, X., Hunter, S. W., Brennan, P. J., Rea, T. H. and Modlin, R. L. A major T-cell antigen of Mycobacterium leprae is the 10 kD heat shock cognate protein. J. Exp. Med. 175(1992)275-284.
16. Mendez-Samperio, P., Lamb, J., Bothamley, G., Stanley, P., Ellis, C. and Ivanyi, J. Molecular study of the T cell repertoire in family contacts and patients with leprosy. Eur. J. Immunol. 142(1988)3599-3604.
17. Mustafa, A. S., Gill, G., Nerland, A., Britton, W. J., Mehra, V., Bloom, B. R., Young, R. A. and Godal, T. Human T-cell clones recognise a major M. leprae protein antigen expressed in E. coli. Nature 319(1986)63-66.
18. Ottenhoff, T. H. M., Klatser, P. R., Ivanyi, J., Elferink, D. G., de Wit, M. Y. L. and de Vries, R. R. P. Mycobacterium leprae -specific protein antigens defined by cloned human helper T cells. Nature 319(1986)66-68.
19. Ottenhoff, T. H. M., Converse, P. J., Gebre, N., Wondimu, A., Ehrenberg, J. P. and Kiessling, R. T-cell reponses to fractionated Mycobacterium leprae antigens in leprosy; the lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur. J. Immunol. 19(1989)707-713.
20. Pal, P. G. and Horwitz, H. A. Immunisation with the extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substabtial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60(1992)4781-4792.
21. Res, P. C. M., Thole, J. E. R. and de Vries, R. R. P. Heat-shock proteins and autoimmunity in humans. Curr. Opin. Immunol. 13(1991)81-98.
22. Rinke de Wit, T. F., Bekelie, S., Osland, A., Miko, T. L., Hermans, P. W. M., van Soolingen, D., Drifhout, J.-W., Schoningh, R., Janson, A. A. M. and T hole, J. E. R. Mycobacteria contain two groEL genes: the second Mycobacterium leprae groEL gene is arranged in an operon with groES. Mol. Microbiol. 6(1992)1995-2007.
23. Rinke de Wit, T. F., Bekelie, S., Osland, A., Wieles, B., Janson, A. A. M. and Thole, J. E. R. The M. leprae antigen 85 complex gene family: identification of the genes for the 85A, 85C and the related MPT51 proteins. Infect. Immun. 61(1993)3642-3647.
24. Rinke de Wit, T. F., Clark-Curtiss, J. E., Abebe, F., Kolk, A. H. J., Janson, A. A. M. and Thole, J. E. R. A Mycobacterium leprae- specific gene encoding an immunologically recognized 45 kDa protein. Mol. Microbiol. 10(1993)829-838.
25. Sathish, M., Esser, R. E., Thole, J. E. R. and Clark-Curtiss, J. E. Identification and characterization of antigenic determinants of Mycobacterium leprae that react with antibodies in sera of leprosy patients. Infect. Immun. 58(1990)1327-1336.
26. Sela S., Thole, J. E. R., Ottenhoff, T. H. M. and Clark-Curtiss, J. E. Identification of Mycobacterium leprae antigens from a cosmid library: characterization of a 15-kilodalton antigen that is recognized by both the humoral and cellular immmune systems in leprosy patients. Infect. Immun. 59(1991)4117-4124.
27. Thole, J. E. R, van Schooten, W. C . A., Keulen, W. J., Hermans, P. W. M., Janson, A. A. M., de Vries, R. R. P. and van Embden J. D. A. Use of recombinant antigens expressed in Escherichia coli K-12 to map B-cell and T-cell epitopes on the immunodominant 65 kilodalton protein of Mycobacterium bovis BCG. Infect. Immun. 56(1988)1633-1640.
28. Thole, J. E. R., Schoningh, R., Janson, A. A. M., Garbe, T., Cornelisse, Y. E., Clark-Curtiss, J. E., Kolk, A. H. J., Ottenhoff, T. H. M., de Vries, R. R. P. and Abou-Zeid, C Molecular and immunological analysis of a fibronectin binding protein antigen secreted by Mycobacterium leprae. Mol. Microbiol. 6(1992)153-163.
29. Trudeau, E. L. Two experiments in artificial immunity against tuberculosis. Trans. Natl. Assoc. Study Prev. Tuberc. 1(1905)157-163.
30. Van Eden, W., Thole, J. E. R., van der Zee, R., Noordzij, A., Van Embden, J. D. A., Hensen, E.J. and Cohen, I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature 331(1988)171-173.
31. Wieles, B., van Agterveld, M., Janson, A., Clark-Curtiss, J. E., Rinke de Wit, T. F., H arboe, M. and Thole, J. E. R. Characterization of a Mycobacterium leprae antigen related to the secreted Mycobacterium tuberculosis protein MPT32. Infect. Immun. 62(1994)252-258.
32. Young, D. B. and Garbe, T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res. Microbiol. 142(1991)55-65.
33. Young, D. B., Kaufmann, S. H. E., Hermans, P. W. M. and Thole, J. E. R. Mycobacterial protein antigens: a compilation. Mol. Microbiol. 6(1992)133-145.
1. Ph.D.; Department of Immunohaematology andBloodbank, University Hospital Leiden, P. 0. Box 9600, 2300 RC Leiden, The Netherlands.
2. M.D.; Department of Immunohaematology andBloodbank, University Hospital Leiden, P. 0. Box 9600, 2300 RC Leiden, The Netherlands.
3. Ph.D.; Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia. E. J. Shannon, Ph.D.,Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.
4. Ph.D.; Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia. E. J. Shannon, Ph.D.,Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.
5. Ph.D.; Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia. E. J. Shannon, Ph.D.,Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.
6. B.Sc.; Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia. E. J. Shannon, Ph.D.,Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.
7. B.Sc.; Department of Medical Microbiology, UniversityCollege London Medical School, Riding House Street, London WI P 7PN, U.K.
8. Ph.D.; Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.
9. Leprosy Control Division of ALERT (ALC), P. 0. Box 165,Addis Ababa, Ethiopia. 10. Ph.D., Department of Medical Statistics, University Hospital Leiden, P. 0.Box 9600, 2300 RC Leiden, The Netherlands.
10. Ph.D.,Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State Universit, P. 0. Box 25072,Baton Rouge, Louisiana 70894, U.S.A.er Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia.
11. M.D., Department of Immunohaematology andBloodbank, University Hospital Leiden, P. 0. Box 9600, 2300 RC Leiden, The Netherlands.
12. D. Frommel, M.D., Ph.D.;.Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia.
13. T. F. Rinke de Wit, Ph.D.,Armauer Hansen Research Institute (AHRI) P. 0. Box 1005, Addis Ababa, Ethiopia.
Reprint requests to J. E. R. Thole, Ph.D., Department of Medical Microbiology, St. Mary's Hospital Medical School, Norfolk Place, London W2 1PG, U.K. FAX = 44-71-2626299.
Received for publication on 30 January 1995.
Accepted for publication in revised form on 23 May 1995.