- Volume 63 , Number 3
- Page: 381–90
Immunogenic properties of the M. leprae recombinant 18-kda antigen purified f rom saccharomyces cerevisiae; enhancement of delayed-type hypersensitivity after gamma-irradiation
ABSTRACT
In this paper we report the purification and study of the immunogenic properties of the Mycobacterium leprae 18-kDa protein antigen produced and secreted by the yeast Saccharomyces cerevisiae, using an expression system we recently described [Biotech. Lett. 16 (1994) 1241-1246]. The 18-kDa protein was purified f rom the yeast culture media by precipitation, ion exchange chromatography (MonoQ) and exclusion size chromatography (Sephacryl S-100). The biological properties of the recombinant protein, previously irradiated with gamma rays, were assayed by immunization of mice. Humoral and cellular responses, monitored by antibody gamma-irradiation of the recombinant protein prior to the administration was shown to significantly potentiate the T-cell response. The data suggest that this irradiated recombinant antigen could be used in a more sensitive standardized skin test to monitor M. leprae infection.RÉSUMÉ
Nous rapportons dans ce papier la purification et l'étude des propriétés immunogéniques de l'antigène protéique de 18-kDa de Mycobacterium leprae produit et sécrété par la levure Saccharrnyces cerevisiae, en utilisant un système d'expression que nous avons récemment décrit [Biotech. Lett. 19 (1994) 1241-1246]. La protéine de 18-kDa fut purifiée du milieu de culture de levure par précipitation, chromatographic d'échange ionique (MonoQ) et chromatographie d'exclision pour la taille (Sephacryl S-100). Les propriétés biologiques de la protéine recombinante, irradiée auparavant par des rayons gamma, ont été testées par immunisation de souris. On a obtenu les réponses humorales et cellulaires, monitorées respectivement par la production d'anticorps et par l'hypersensibilité retardée. De plus, on a montré que l'irradiation gamma de la protéine recombinante avant l'administration stimulait la réponse des cellules T de manière significative. Les données suggèrent que cet antigène recombinant irradié pourrait ê;tre utilisé dans un test cutané standardisé plus sensible pour la surveillance de l'infection lépreuse.RESUMEN
En este trabajo reportamos la purificación y el estudio de las propiedades inmunogénicas de la proteina de 18 kDa de Mycobacterium leprae producida y secretada por la levadura Saccharomyces cerevisiae usando un sistema de expresión recientemente descrito por nosotros [Biotech, Lett. 16(1994) 1241-1246]. La proteina de 18 kDa fue purificada del medio de cultivo de la levadura por precipitación, cromatografía de intercambio iónico (MonoQ), y por cromatografia de exclusión por tamaño (Sephacryl S-100). Las propiedades biológicas de la proteina recombinante irradiada con rayos gamma, se analizaron por ensayos de inmunización de ratones; las respuestas humoral y celular generadas, se visualizaron por producción de anticuerpos y por reacciones de hipersenbilidad tardía, respectivamente. Se encontró que la irradiación gamma de la proteína recombinante antes de su administración, potenció significativamente la respuesta mediada por células T. Los datos sugieren que este antigeno recombinante irradiado podría usarse en pruebas dérmicas (previa estandarización de las mismas) para establecer la infección por M. leprae.The use of irradiated biological material for immunization has been a field of growing interest in recent years. Irradiation is a practical and nonexpensive way to sterilize biological materials (26). Furthermore, radiation-induced modifications in the structure of carbohydrates and proteins might cause the host immune system to mount an effective response against molecules which, in their normal conformation, are only weak immunogens (39). For this purpose, the irradiation must strike an ideal balance, producing antigens that depart sufficiently from the normal conformation to induce potent immunity yet, at the same time, resemble the original native structure sufficiently for immunological memory to be reactivated upon exposure to normal challenge infection (39). Actually, irradiated proteins have been used in the immunization of laboratory animals against protozoans, helminths, and nematodes, as recently reviewed (39). In humans, sucessful immunization of humans against malaria was accomplished using irradiated Plasmodium (9,19).
Mycobacterium leprae, the etiologic agent of leprosy, can only be obtained in large quantities from experimentally infected armadillos. Irradiated M. leprae from armadillos is currently the major source of this mycobacterium (18). Inactivated mycobacteria are used for preparation of skin-test antigens and experimental vaccines in clinical trials (34,40). The inactivation of mycobacteria can be performed by autoclaving or gamma irradiation at a 2.5 Mrad dose (34). Many studies have confirmed that heat-killed, human-derived and irradiated armadillo-derived M. leprae are equivalent as antigens for skin tests and both are accepted by the World Health Organization (WHO)(24,36). However, the presence of armadillo contaminants in this preparation was recently confirmed (32) and previous studies have indicated that human contaminants also can be found (23).
Given that different M. leprae antigens are now available (18), such problems could be overcome using, instead of the whole mycobacterium, purified antigens presenting relevant immunological properties (15).
Among the known M. leprae protein antigens, the 18-kDa heat-shock protein (p18) has deserved special attention since it was found to stimulate both murine and human T-cells (11,17,31). It was first shown that this antigen is recognized by pooled sera from human patients with lepromatous leprosy (7). Subsequently, as a monoclonal antibody against this protein became available (L5), it was clear that B-cell epitopes also are recognized by mice (14). Finally, antibodies against this antigen were found in human patients with leprosy and tuberculosis (20).
Most importantly, since resistance against leprosy is almost entirely mediated by cellular immune responses, there also is consistent evidence of the participation of p18 in human cell-mediated immunity. This protein is recognized by M. leprae -specific, CD4+ T-cell clones isolated from healthy vaccinated individuals immunized with killed M. leprae (28), in the context of HLADR4, HLA-DW4, and HLA-DR1, meeting therefore a requirement to be useful as a diagnostic reagent or vaccine (29). Long-lasting T-cell reactivity toward p18 was demonstrated by an analysis of T-cell clones obtained from these vaccinated individuals 8 years later (30). Moreover, peripheral blood mononuclear cells from 93% of long-term leprosy contacts, 50% of tuberculoid leprosy patients, and 70% of BCG-vaccinated individuals were shown to proliferate in the presence of p 18, with a stronger response in the contacts, thus suggesting that p18 could have a protective role (11).
Given the difficulties of in vitro cultivation of M. leprae, the cloning of the major antigen genes was performed by different groups, looking for expression of sufficient amounts of protein antigens (4, 5, 38, 41) In our group, a new system for efficient expression of the 18-kDa M. leprae antigen in the yeast Saccharomyces cerevisiae was established. In this system, gene expression is regulated by the hybrid yeast promoter GAPDH/ADH2 and the gene product is secreted into the culture medium. The recombinant protein yield was 140 mg per liter and degradation was negligible after adjustments of culture media composition and PH (33).
Although some of the immunogenic properties of different recombinant M. leprae p18 were analyzed previously in animal models (2,12,13,17), the occurrence of differences in the post-translational protein processing between the expression hosts and M. leprae could result in some structural differences in the recombinant antigen. Therefore, the biochemical and immunological properties of every new recombinant antigen must be analyzed carefully to verify the maintenance of the authentic biological properties.
In this paper, we describe the purification of the 18-kDa antigen produced in the yeast expression-secretion system we developed, and we report the usefulness of this purified protein, either untreated or previously gamma-irradiated, to elicit humoral as well as delayed-type hypersensitivity responses in mice.
MATERIALS AND METHODS
Yeast culture
A pLep2 transformant clone of the S. cerevisiae strain JSC310 (MATa, leu 2-3, 112, ura 3-52, pep 4-3, prb 1-1122, prc 1-407:: DM15 (pGAP/ADRl::G418r), cirº) that expresses and secretes the 18-kDa protein of M. leprae (p18) (33) was grown for 48 hr at 30ºC in 500 ml of minimal medium (SD) with 8% dextrose and without leucine. The cells were then transferred to 10 flasks containing 1 liter of YM (YPD 3.5% + yeast nitrogen base 0.67%) buffered to pH 6.0 with 1% potassium succinate, and growth was carried out for 48 hr at 30ºC. The cells were precipitated by centrifugation at 3000 x g x 30 min in a Beckman J6-B centrifuge, and the supernatant was retained for further purification. Yeast culture media are detailed elsewhere (1).
Protein purification
Precipitation. The medium was first clarified by adding 0.1 M citric acid to adjust the pH to 5.0 and centrifuging at 12,000 x g x 20 min. The remaining proteins were precipitated by the addition of ammonium sulfate to 60% (w/v) and centrifuged as described above.
Chromatography. Each precipitate was redissolved in TE (TrisHCl 10 mM, EDTA 1 mM pH 8.0), the pH was adjusted to 7.5, and conductivity to less than 10 milli-Siemens per centimeter. The solution was passed through a MonoQ column previously equilibrated with TE under a gradient of 0 to 1 M NaCl using FPLC (Pharmacia).
The fractions containing the protein were pooled and chromatographed in a Sephacryl S-100 molecular exclusion column previously equilibrated with TE at room temperature using FPLC (Pharmacia).
Protein analysis. All fractions and pools were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (15 or 17.5%) after each purification step. The final product obtained was quantitated by the Bradford method. Densitometer analysis of SDS-PAGE was performed in an Ultroscan XL (Biorad) equipment. These procedures are detailed elsewhere (1).
Immunological properties of p18
Immunological assays were performed as described elsewhere (10), with slight modifications. Animals. Pathogen-free CBA/J mice (H-2 complex: Kk, Abk Aak Ebk Eak Dk; other genetic loci: Thy-12, CD51, CD82, CD452) (10), weight 25 g to 28 g, were raised at the Instituto de Medicina Tropical, Universidade de Sao Paulo.
Mice were separated in groups of 5 animals each, and immunized with: I-recombinant p18, II-irradiated recombinant p18, III-alum, IV-saline, V-Mitsuda.
Antigens. Recombinant p18. Recombinent p18 was prepared as follows: 250 µ l of p18 (0.5 mg/ml) were mixed with 100 µ lof NaHC03 and 250 µ l of A1K(S04)2.
Irradiated recombinant p18. The same p18 solution was irradiated in a Gammacell 220 60 Co source (Atomic Energy of Canada Ltd.) at a 2000 Gy dose, in the presence of oxygen, at room temperature. Immunizations were carried out with a mixture prepared as described for the recombinant p18.
Mitsuda. A solution with the same protein concentration (0.5 mg/ml) was prepared with the antigen prepared at the Section of Mycobacteria of the Instituto Adolfo Lutz (lot 04/31.VII.92), a generous gift of Dr. Moisés Palacci. This antigen was prepared according to the procedures and safety requirements described by the World Health Organization (40). Immunizations were performed in a mixture with alum prepared as described above.
All antigens were administered intradermally in the backs of mice, in a volume of 50 µ l (10 µ g) at days 0 and 14. The animals were bled at days 21 and 42. The presence of specific anti-pl8 antibodies was monitored by ELISA and Western blot.
ELISA. Microplates Maxisorp (Nunc) were coated at 2 ng/well of p18 in 0.05 M carbonate/bicarbonate buffer pH 9.6 for 2 hr at room temperature, and then incubated with the mice sera for 1 hr at 37ºC at dilutions from 10-1 to 10-6. The wells were then incubated for 1 hr at 37ºC with anti-mouse horseradish peroxidase conjugate (Tago), and the reaction was developed by the addition of orthophenylenediamine and H2O2 . The optical density (OD) at 492 nm was measured in a Titertek Multiscan Plus MK II ELISA plate reader.
Western blot. Proteins precipitated by trichloroacetic acid (TCA) from S. cerevisiae JSC310/pLep2 culture medium were electrophoresed in a 17.5% SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then incubated with murine sera diluted 1/100 for 2 hr at room temperature, then 1 hr with anti-mouse horseradish peroxidase conjugate 1/500 (Tago). The reaction was developed by diaminobenzidine and H2O2.
Delayed-type hypersensitivity (DTH). DTH was monitored by foot pad enlargement (FPE). The animals were challenged at 28 days with 5 µg of recombinant (left hind foot) or irradiated recombinant p18 (right hind foot). The thickness of the foot was measured with a manual micrometer (Mitutoyo do Brasil) just prior to and 24, 48 and 72 hr after injection of the antigen. For corrected FPE, the previous foot pad measurement was subtracted.
Statistical analysis
Statistical analysis of the results was carried out using the two-tailed Student's t test.
RESULTS
Protein purification
After 48 hr growth of S. cerevisiae strain JSC310/pLep2, the supernatant was separated from the cells and analyzed by 15% SDS-PAGE. As shown in Figure 1, the major protein species in the supernatant was p18, although a lower band was also observed due to some degradation during culture.
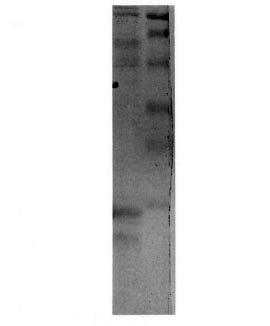
Fig . 1. SDS-PAGE 15% analysis of the proteins inthe supernatant of a 48-hr culture of yeast strain JSC310/pLep2 (left lane). Right lane = molecular weight stan-dards 106,000, 80,000, 49,500, 32,500, 27,500, 18,500.
A small pellet was obtained upon precipitation at the isoeletric point. This pellet was resuspended in 6 ml of TE. Since most of the p18 was still found in the supernatant, an ammonium sulfate precipitation step was carried out and the pellet was resuspended in 400 ml of TE and dialyzed for 48 hr against several changes of TE.
The solution was sequentially applied to a MonoQ column and eluted by 0-1 M NaCl gradient. The elution profile is shown in Figure 2. The fractions eluting around 0.3 M NaCl contained the p18. Thirty-five runs were carried out and the p 18 fractions were pooled.
Fig. 2. Elution profile from ion exchange chro-matography on MonoQ. Horizontal axis = eluting fractions collected; left vertical axis = transmittance at 280nm (T, normal line); right vertical axis = NaCl molarconcentration (M, bold line). Bar = fractions pooledfor further purification.
After Sephacryl S-100 chromatography, p18 was found to be spread through many different fractions where high molecular weight proteins were also present. The most pure p18 fractions were pooled and concentrated by ultrafiltration. Finally, 5.2 mg of purified p18 were obtained in a volume of 2.2 ml, with a final yield of 0.52 mg of purified protein per liter of culture. The SDS-PAGE profile of the final product is shown in Figure 3. The purity of the preparation was estimated to be around 95% by densitometer analysis.
Fig. 3. SDS-PAGE 17.5% analysis of purified p18(right lane). Left lane = molecular weight standards 43,000, 29,000, 18,400, 14,300, 6200, 3000.
Immunogenic properties of p18 in mice
Serology. The induction of humoral response in mice CBA/J was monitored by measuring antibody titers by ELISA at 21 and 42 days after immunization. As shown in Table 1, 21 days after immunization the OD at 492 nm from animals immunized with recombinant or irradiated recombinant pi 8 were higher than the control groups and the Mitsuda-immunized group. Specific anti-pl8 antibodies were detected to titers of 10-5. Similar results were found 42 days after immunization (data not shown). No statistically significant difference was observed between the humoral response of recombinant or irradiated recombinant p18 immunized animals, although a slightly higher absorbance was detected in mice immunized with the irradiated protein at day 21.
To verify that the antibodies detected by ELISA were directed toward p18 and not to any possible yeast contaminant, sera were also assayed by Western blot (Fig. 4). Immunized animals' sera (lanes 2 and 3) recognized the same bands recognized by the specific L5 monoclonal antibody (lane 1), while no bands were detected in the control groups (lanes 4 and 5). The higher band was found at the expected molecular weight and was associated with a lower band, probably a p18 degradation product arising during culture.
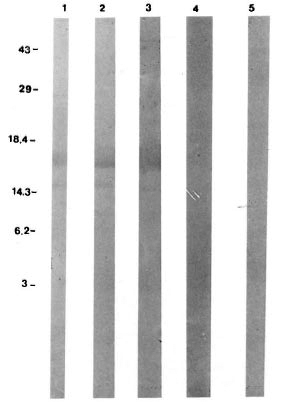
Fig. 4. Western blot analysis of CBA/J mice anti-p18 antibodies 21 days after immunization. Molecular weight standard positions in kDa shown at left. Lane1 = positive control (L5 monoclonal antibody); lane 2 = animal immunized with recombinant p18; lane 3 = animal immunized with irradiated recombinant p18; lane 4 = animal immunized with alum; lane 5 = animalimmunized with saline.
Delayed-type hypersensitivity
Corrected FPE measured 48 hr after challenge was statistically higher in animals immunized with the recombinant protein than the controls. Furthermore, irradiated recombinant p18 gave much higher FPE than did all the other groups, including those animals immunized with the recombinant protein (Table 2).
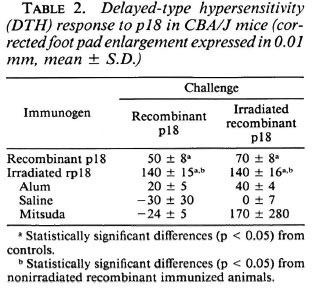
DISCUSSION
In our expression system, the M. leprae 18-kDa gene was cloned in fusion with the S. cerevisiae α factor leader sequence, thus allowing the 18-kDa protein to be secreted by the yeast cells harboring the recombinant plasmid. This construct produced the antigen as a full-length protein, not a fusion protein (33). Therefore, the purification procedures were facilitated since the 18-kDa protein was found in the culture supernatant. However, this recombinant protein proved to be extremely sensitive to acid degradation. When cells were grown in unbuffered media, all the secreted protein appeared to be degraded. Using buffered media, however, this degradation was significantly reduced, although still occurring at pH 6.0.
The yield of the purification (0.52 mg/1 of culture) was low when compared with the expression yield (33). This was due to a number of different reasons: first, degradation during culture and, second, spreading of the protein through the column during the last purification step. It was concluded that the protein aggregates and may bind to some high-molecular-weight yeast proteins. Nevertheless, the degree of purity of this preparation (ca. 95%) allowed the development of different immunological assays to verify whether this recombinant protein was capable of inducing humoral and/or cellular responses, using CBA/J mice as a model.
The purified yeast-derived p18 was used for immunization of CBA/J mice. In contrast to published reports, we adopted a protocol for immunization that would mimic the conditions to be used in humans. For this reason, the recombinant p18 was administered in the back (subcutaneous route) and not at the base of the tail (intravenous route) of the mice. Furthermore, although alum was reported as a poor adjuvant in the induction of cellular responses (3), it was preferred because incomplete Freund's adjuvant cannot be used in humans. Using this protocol, specific anti-p18 antibodies were detected to titers of10-5 up to 42 days after immunization. The possibility that the antibodies detected by ELISA were directed to some yeast residual contaminant was discarded by the results of the Western blot analysis, since immunized animals' sera recognized the same bands as the specific L5 monoclonal antibody. No significant difference in the humoral response was observed using gamma-irradiated p18.
Delayed-type hypersensitivity (DTH) was assayed by mouse foot pad enlargement. A strong DTH response was observed, which could still be enhanced threefold upon previous gamma-irradiation of the antigen. This effect might be associated with structural or amino acid changes induced by radiation (37) that would render this protein more effectively recognized by the immune system. We may speculate about still another potential advantage of protein irradiation, especially against obligately intracellular macrophage parasites, such as the mycobacteria. Indeed, free radicals and reactive oxygen species mediate the protein modifications induced by irradiation (37), being as well the major mediators of protein damage inside the macrophages. In this way, irradiated proteins could mimic structures of the mycobacterium that would be seen only after interaction with the host macrophages.
To our knowledge, this is the first report of in vivo DTH induction using yeast-derived p18. Most of the studies reported in the literature examined the T-cell response to p18 using in vitro assays (11,17). Here we used the DTH response to monitor the activity of Th1 cells (8), which are the cells directly involved in the development of cellular immune responses. Furthermore, it was recently shown that different Th1 subsets are involved in in vitro lymphoproliferative assays and in vivo DTH assays using the 18kDa M. leprae protein (2). Bäcksträm, et al. (2) reported a decrease in DTH response when more than one dose of Escherichia coli -derived p18 was inoculated into mice. In our case, a lower dose of yeast-derived p18 was used in two immunizations, and high antibodies titers were obtained simultaneously with a strong DTH response. However, these discrepant results could be associated with differences in dose, time, or adjuvant used, besides the origin of the antigen.
Skin tests measuring the DTH response have an important role in the management of leprosy patients. These tests are routinely used in the classification of leprosy for individual patients and should be used to evaluate protective response after trials using new antileprosy vaccines (18). Currently, such a test is performed with Mitsuda antigen obtained from lepromatous leprosy lesions in humans or from experimentally infected armadillos (40). These antigen sources are very irregular, expensive, and potentially hazardous (18). The essence of the protocol for preparation of this antigen is the application of relatively gentle separation methods to assure the preservation of the immunogenicity (40). Thus, it is not surprising that the presence of armadillo-derived proteins in such preparations recently was reported (32), in addition previous data also suggested the presence of human skin components interfering with the immune response (23). The use of recombinant antigens to replace the former antigens would allow a broader utilization of skin tests and the use of preparations that could be easily standardized. Our experiments indicate a potential usefulness of the yeast recombinant p18 as an antigen in human skin tests, given that a DTH response was elicited by the recombinant p18, which could still be enhanced considerably by previous irradiation of the antigen.
Interestingly, the purified p18 and the Mitsuda antigen did not crossreact, either at the humoral, or at the cellular level in the experiments described here since no anti-pi 8 was produced after immunization with the Mitsuda antigen. The same results were obtained in another experiment in which p18 was used as the immunogen and challenge was performed with the Mitsuda antigen (data not shown). These results could be explained by the paucity of p18 in the Mitsuda antigen preparation, since it was recently shown that the 18-kDa protein is a minor constituent of M. leprae, its isolation directly from M. leprae being impossible (35). On the other hand, the Mitsuda antigen is prepared by autoclaving, mechanical homogenization, filtration, and dilution in 5% phenol (40). These treatments could be responsible for the removal or destruction of the M. leprae p18. Still another possibility is that response to p18 is not an early event after immunization, as recently shown in household leprosy contacts (22).
The M. leprae 18-kDa antigen formerly was considered to be restricted to two species of mycobacteria since the anti-pl8 specific monoclonal antibody was found to crossreact only with an 18-kDa protein from M. habana, now known as M. simiae serovar 1 (21). More recently, DNA hybridization studies using the entire 18-kDa gene as a probe demonstrated the existence of 18kDa homologs in several other mycobacteria (25). In spite of previous immunological evidence suggesting the presence of this gene also in M. tuberculosis (11,20), no homologous gene was found, even using polymerase chain reaction with degenerate consensus primers (6). At the human T-cell level, there is evidence of crossreaction with M. scrofulaceum (28), Mycobacterium w (27), and M. bovis BCG (11,15). However, in the latest report by Mustafa, et al. (29), BCG-induced T-cell lines did not respond to p18, supporting earlier findings of M. leprae specificity of p18 with human M. leprae- induced T cells. In fact, in this study, M. leprae- induced T-cell lines from four of the five M. leprae- vaccinated patients responded to p18 (29).
Even with the lack of sufficient specificity, the use of a mixture of purified proteins would allow the development of improved, standardized, skin-test reagents when compared to human- or armadillo-derived mycobacteria. Based on our data and on the evidence showing the presence of long-lasting cellular response to this protein in leprosy contacts and patients, the M. leprae 18kDa antigen would be an important candidate component of such a mixture, especially after previous gamma irradiation.
In a recent work, Gelber, et al. (16) showed that no single M. leprae antigen was capable of inducing long-lasting protection against M. leprae multiplication in mice. Therefore, they suggested that for the murine model of leprosy, as well as for potential human applications, vaccination should include multiple M. leprae proteins or even subfractions of soluble proteins. It remains to be seen whether the 18-kDa protein would be a relevant component in such a mixture.
Acknowledgment. This work was supported by grants from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, from Centro Brasilciro-Argcntino de Biotecnologia and from LIM-HC-FMUSP. We would like to acknowledge Almir Robson Ferreira (IMT) for preparation of the illustrations.
REFERENCES
1. Ausubel , F. M., Brent, R., Kingston, R. E., Moore , D. D., Seidman , J. G., Smith , J. A. and Struhl, K., eds. Current Protocols in Molecular Biology. New York: John Wiley & Sons, 1987.
2. Bäckström , T. M., Harris , D. P., Prestidge, R. L. and Watson , J. D. Genetic control of immune response to the 18-kDa protein of Mycobacterium leprae: different Th1 subsets may be involved in proliferative and dclayed-type hypersensitivity responses. Cell. Immunol. 142(1992)264-274.
3. Bomford, R. The comparative selectivity of adjuvants for humoral and cell-mediated immunity. I. Effect on the antibody response to bovine serum albumine and sheep red blood cells of Freund's incomplete and complete adjuvants, alhydrogel, Corynebacterium parvum, Bortedella pertussis, muramyl dipeptide and saponin. Clin. Exp. Immunol. 39(1980)426-431.
4. Booth, R. J., Grandison, P. M., Prestidge, R. L. and W atson , J. D. The use of a 'universal' yeast expression vector to produce an antigenic protein of Mycobacterium leprae. Immunol. Lett. 19(1988)65-73.
5. Booth, R. J., H arris , D. P., Love , J. M. and Watson , J. D. Antigenic properties of Mycobacterium leprae: complete sequence of the gene for the 18-kDa protein. J. Immunol. 140(1988)597-601.
6. Booth, R. J., W illiams , D. L., Moudgil, K. D., Noonan , L. C, G randison, P. M., McKee , J. J., Prestidge, R. L. and Watson , J. D. Homologs of Mycobacterium leprae 18-kilodalton and Mycobacterium tuberculosis 19-kilodalton antigens in other mycobacteria. Infect. Immun. 61(1993)1509-1515.
7. Britton , W. J., Hellqvist , L., Basten , A. and Raison, R. L. Mycobacterium leprae antigens involved in human immune responses: I. Identification of four antigens by monoclonal antibodies. J. Immunol. 135(1985)4171-4177.
8. Cher , D. J. and Mossman , T. R. Two types of murine helper T cell clone: II. Delayed type hypersensitivity is mediated by Th1 clones. J. Immunol. 138(1987)3688-3694.
9. Clyde, D. F. Immunity to falciparum and vivax malaria induced by irradiated sporozoitcs: a review of the University of Maryland studies, 1971-75. Bull. WHO 68suppl.(1990)9-12.
10. Coligan, J. E., Kruisbeck, A. M., Margulies, D. H., Shevach, E. M. and Strober, W., eds. Current Protocols in Immunology. New York: John Wiley & Sons, (1992).
11. Dockrell, H. M., Stoker, N. G., Lee, S. P., Jackson, M., Grant, K. A., Jouy, N. F., Lucas, S. B., Hasan, R., Hussain, R. and McAdam, K. P. W. J. T-Cell recogniton of the 18-kilodalton antigen of Mycobacterium leprae. Infect. Immun. 57(1989)1979-1983.
12. Doherty, T. M., B ackstrom, B. T., Prestidge, R. L., Love, S. G., Harding, D. R. K. and W atson, J. D. Immune responses to the 18-kDa protein of Mycobacterium leprae: similar B cell epitopes but different T cell epitopes seen by inbred strains of mice. J. Immunol. 146(1991)1934-1940.
13. Doherty, T. M., Booth, R. J., Love, S. G., Gibson, J. J., Harding, R. K. and Watson, J. D. Characterization of an antibody-binding epitope from the 18kDa protein on Mycobacterium leprae. J. Immunol. 142(1989)1691-1695.
14. Engers, H. D., Abe, M., Bloom, B. R., Mehra, V., Britton, W., Buchanan, T. M., Khanolkar, S. K., Young, D. B., Closs, O., Gillis, T., Harboe, M., I vanyi, J., Kolk, A. H. J. and Shepard, C. C. Results of a World Health Organization-sponsored workshop on monoclonal antibodies to Mycobacterium leprae. Infect. Immun. 48(1985)603-605.
15. Estrada-G ., I. C. E., Gutierrez, M. C, Esparza, J., Quesada-Pascual, F., Estrada-Parra, S. and Possani, L. D. Use of synthetic peptides corresponding to sequences of Mycobacterium leprae proteins to study delayed-type hypersensitivity response in sensitized guinea pigs. Int. J. Lepr. 60(1992)18-27.
16. Gelber, R. H., M ehra, v., B loom, B., Murray, L. P., Siu, P., T sang, M. and Brennan, P. J. Vaccination with pure Mycobacterium leprae proteins inhibits M. leprae multiplication in mouse foot pads. Infect. Immun. 62(1994)4250-4254.
17. Harris, D. P., Bäckstrom, B. T., Booth, R. J., Love, S. G., Harding, D. R. and Watson, J. D. The mapping of epitopes of the 18kDa protein of Mycobacterium leprae recognized by murine T cells in a proliferation assay. J. Immunol. 143(1989)2006-2012.
18. Hastings, R. C, Gillis, T. P. K rahenbuhl, J. L. and F ranzblau, S. G. Leprosy. Clin. Microbiol. Rev. 1(1988)3330-3348.
19. Herrington, D., D avis, J., Nardin, E., Beier, M., Cortese, J. F., Eddy, H., Losonsky, G., Hollingdale, M., Steinz, M., Levine, M., Nussenzweig, R. S., Clyde, D. and Edelman, R. Successful immunization of humans with irradiated malaria sporozoitcs: humoral and cellular responses of the protected individuals. Am. J. Trop. Med. Hyg. 45(1991)539-547.
20. Hussain, R., Dockrell, H. M., Kifayet, A., Daud, A., Watson, J. D., Chiang, T. J. and S toker, N. G. Recognition of Mycobacterium leprae 18-kDa protein in leprosy. Int. J. Lepr. 60(1992)368-375.
21. Lamb, F. I., Singh, N. B. and Colston, M.J. The specific 18-kilodalton antigen of Mycobacterium leprae is present in Mycobacterium habana and functions as a heat-shock protein. J. Immunol. 144(1990)1922-1925.
22. Launois, P., N'Diaye, M. N., Sarthou, J. L., Drowart, A., Van Vooren, J. P., Cartel, J. L. and Huygen, K. T cell reactivity against antigen 85 but not against the 18- and 65-kD heat shock proteins in the early stages of acquired immunity against M. leprae. Clin. Exp. Immunol. 96(1994)86-90.
23. Leiker, D. L. Studies on the lepromin test: I. Influence of the bacillary and tissue components in dilutions of lepromin. Int. J. Lepr. 29(1961)157-167.
24. Meyers, W. M., Kvernes, S. and Binford, C. H. Comparison of reactions to human and armadillo lepromins in leprosy. Int. J. Lepr. 43(1975)218-233.
25. Moudgil, K. D., Williams, D. L. and Gillis, T. P. DNA hybridization analysis of mycobacterial DNA using the 18-kDa protein gene of Mycobacterium leprae. FEMS Microbiol. Immunol. 89(1992)165-174.
26. Murray, D. R. Biology of Food Irradiation. Somserset, U.K.:Research Studies Press, Ltd., 1990.
27. Mustafa, A. S. Identification of T-cell activating recombinant antigens shared among three candidate antileprosy vaccines, killed M. leprae, M. bovis BCG and Mycobacterium w. Int. J. Lepr. 56(1988)265-273.
28. Mustafa, A. S., G ill, H. K., Nerland, A., Britton, W. J., Mehra, V., Bloom, B. R., Young, R. A. and Godal, T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature 319(1986)63-66.
29. Mustafa, A. S., L undin, K. E. A. and Oftung, F. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLADR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect. Immun. 61(1993)5294-5301.
30. Mustafa, A. S. and Oftung, F. Long lasting T cell reactivity to Mycobacterium leprae antigens in human volunteers vaccinated with killed M. leprae. Vaccine 11(1993)1108-1118.
31. Oftung, F., Shinnick, T. M., Mustafa, A. S., Lundin, K. E. A., Godal, T. and Nerland, A. H. Heterogeneity among human T cell clones recognizing an HLA-DR4, Dw4-restricted epitope from the 18-kDa antigen of Mycobacterium leprae defined by synthetic peptides J. Immunol. 144(1990)1478-1483.
32. Pessolani, M. C. V., Hunter, S. W. and Brennan, P. Relationship between host histones and armadillo-derived Mycobacterium leprae. Int. J. Lepr. 61(1993)381-388.
33. Pinho, J. R. R., Cardi, B. A., Andrade, H. F., Jr., Barr, P. J., Bathurst, I. C. and Schenberg, A. C. Expression of the 18kDa protein of Mycobacterium leprae in Saccharomyces cerevisiae. Biotech. Lett. 16(1994)1241-1246.
34. Ponninghaus, J. M. and Fine, P. E. M. Sensitization studies with potential leprosy vaccine preparations in northern Malawi. Int. J. Lepr. 54(1986)25-37.
35. Rivoire, B., Pessolani, M. C. V., Bozic, C. M., Hunter, S. W., Hefta, S. A., Mehra, V. J. and Brennan, P. J. Chemical definition, cloning, and expression of the major protein of the leprosy bacillus. Infect. Immun. 33(1994)2417-2425.
36. Shepard, C. C, Draper, P., Rees, R. J. W. and Lowe, C. Effect of purification steps on the immunogenicity of Mycobacterium leprae . Br. J. Exp. Pathol. 61(1980)376-379.
37. Sonntag, C. The Chemical Basis of Radiation Biology. London: Taylor & Francis Ltd., 1987.
38. Stoker, N. G., Grant, K. A., Dockrell, H. M., Howard, C. R., Jouy, N. F. and McAdam, K. P. W. J. High level expression of genes in phage lambda gt11. Gene 78(1989)93-99.
39. Wales, A. and Kusel, J. R. Biochemistry of irradiated parasite vaccines: suggested models for their mode of action. Parasitol. Today 8(1992)358-363.
40. World Health Organization. Recommended safety requirements for the preparation of lepromin: a WHO memorandum. Bull. WHO 57(1979)921-923.
41. Young, R. A., Mehra, V., Sweetser, D., Buchanan, T., Clark-Curtiss, J., Davis, R. W. and Bloom, B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature 316(1985)450-452.
1. M.D.; (graduate student, Ph.D. program, Instituto de Quimica da Universidade de Sao Paulo).
2. M.Sc.; Instituto Adolfo Lutz, Instituto de Medicina Tropical de Sao Paulo, Divisao de Radiobiologia, IPEN/CNEN and Centro de Pesquisas em Biotecnologia, Universidade de Sao Paulo, Sao Paulo, Brazil.
3. M.D.; Instituto Adolfo Lutz, Instituto de Medicina Tropical de Sao Paulo, Divisao de Radiobiologia, IPEN/CNEN and Centro de Pesquisas em Biotecnologia, Universidade de Sao Paulo, Sao Paulo, Brazil.
4. Ph.D.; Chiron Corporation, Emeryville, California, U.S.A. (currently at LXR Biotechnology, Richmond, California, U.S.A.).
5. Ph.D.; Chiron Corporation, Emeryville, California, U.S.A. (currently at LXR Biotechnology, Richmond, California, U.S.A.).
6. Ph.D.; Instituto Adolfo Lutz, Instituto de Medicina Tropical de Sao Paulo, Divisao de Radiobiologia, IPEN/CNEN and Centro de Pesquisas em Biotecnologia, Universidade de Sao Paulo, Sao Paulo, Brazil.
7. Ph.D.; Instituto Adolfo Lutz, Instituto de Medicina Tropical de Sao Paulo, Divisao de Radiobiologia, IPEN/CNEN and Centro de Pesquisas em Biotecnologia, Universidade de Sao Paulo, Sao Paulo, Brazil.
Reprint requests to Dr. J. R. R. Pinho, Instituto Adolfo Lutz, Av. Dr. Arnaldo 355, 01246-902 Sao Paulo, SP, Brazil.
Received for publication on 14 November 1994.
Accepted for publication in revised form on 16 March 1995.