- Volume 63 , Number 3
- Page: 457–60
Ultrastructural changes in M. leprae passed to laboratory animals
To the Editor:
It is well documented that biological structural changes occur in some infectious agents when passed to different hosts (1,9). Some evidence was obtained (4,5) that some biological properties of Mycobacterium leprae isolated from leprosy patients and passed to mice and rats underwent changes. Adaptation of the original leprosy strain to a new host is usually accompanied by the increased virulence of the pathogen manifested as a shorter incubation period, generalization of the disease, as well as by changes in the spectrum of higher fatty acids
Two ways of division of the mycobacteria were observed by us: a) by growing in of the mycobacterial cytoplasmic membrane only with intact cell wall (in this case a new cell wall at the site of the division is formed by a bacterial cell on the surface of the growing in of the cytoplasmic membrane). After the dividing partition has formed, lysis of the part of the cell wall over the partition occurs and the daughter cells arc separated. Characteristic for this type of division is a nonround shape of mycobacterial poles at the side of the dividing partition on separation of the cells; b) by growing in of the whole envelope as in Figure 2 (cell wall and cytoplasmic membrane). In this case the poles are rounded at the site of the division. It is supposed that the resulting defect of mycobacterial surface, until the completion of the process of division, is filled up with the remains of the microcapsule always present after completion of the mycobacterial division (6,7) and in the antigenic structure of M. leprae (2).
We have found no evidence in the available literature for changes in the ultrastructure of M. leprae repeatedly passed from leprosy patients to laboratory animals although there is an electron microscopic finding (3) of changed forms of M. leprae in mouse foot pads after a single passage according to Shepard (8).
Our present investigation was centered on studying the ultrastructural changes in M. leprae while being passed to mice and nine-banded armadillos.
M. leprae isolated from skin biopsies of five leprosy patients (original strain); from lepromas, spleens, livers and lymph nodes of three armadillos (first passage from the same patients); from infected foot pads of mice using Shepard's method (first to eighth passages); and leprosy bacilli passed to mice from armadillo (second passage from leprosy patients) were the objects of the investigation. M. leprae were taken from the animals within the following time periods from the time of the inoculation: 1.5 years in armadillos, 12-15 months in M. leprae of the first and second passages from mice; 5 months in M. leprae of the third to eighth passages to mice.
Tissue biopsies were cut and fixed in glutaraldehyde-osmium by a standard method, then dehydrated in increasing concentrations of ethanol and propylene oxide, and embedded with epoxy resins. Ultrathin sections of the tissue from mouse foot pads infected with M. leprae taken from a leprosy patient (the first passage) showed intra- and extracellular bacterial cells, the ultrastructure of which differed from the original strain in a reduced microcapsule, a thicker cell wall and a condensed matrix of bacterial cytoplasm that is more peculiar for resting bacterial cells. Mycobacteria located inside the phagocytic cytoplasm were surrounded by electron transparent spaces, so-called "peribacterial zone" peculiar to M. leprae of the original strain as well (Fig. 1).
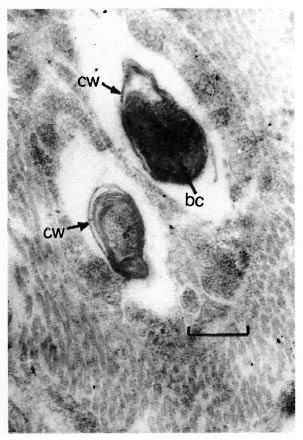
Fig. 1. Electron micrograph of M. leprae of the first passage (patient-to-mouse foot pad), showing thickened cell wall (cw) and condensed bacterial cytoplasmic matrix (bc) (uranyl accate and lead citrate stain; bar =0.3 µ m).
The ultrastructure of M. leprae from the second passage (mouse-to-mouse and armadillo-to-mouse) was similar: the mycobacteria had a fragmentary microcapsule and a cell wall with tightly adjacent cytoplasmatic membrane and increased amounts of cytoplasmic inclusions (so-called "homogeneous bodies"). These strains differed from mycobacteria of the first passage by the abundance of dividing cells, a well-defined nucleoid and a more-developed network of intracytoplasmic membraneous structures (mesosomes). M. leprae of the second passage were dividing in two ways: similar to the way the original strain divides, i.e., by means of growing in of the cytoplasmic membrane only without involving the cell wall being developed in the division process, and through the growing in of the whole envelope (Fig. 2).
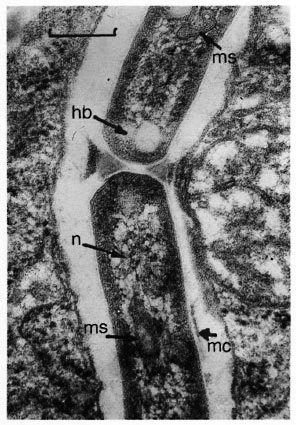
Fig. 2. Electron micrograph of M. leprae of the second passage (mouse-to-mouse), showing a microcapsule (mc), homogeneous bodies (hb), nucleoid (n) and mesosomes (ms). Cells are dividing by means of growing in of the whole envelope (uranyl acetate andlead citrate stain; bar = 0.3 µ m).
For M. leprae strains of the third passage, a great amount of "homogenous bodies" in the cytoplasm and the appearance of intracytoplasmic granules of high electron density (volutin granules) were specific. Numerous dividing cells were found. Again, they divided through the ways peculiar to the cells of the second passage (Fig. 3).
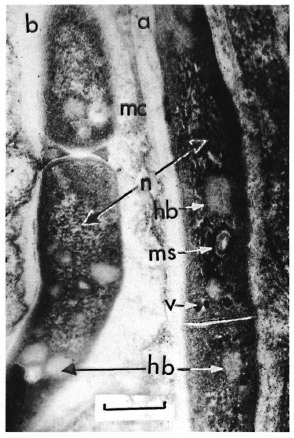
Fig. 3. Electron micrograph of M. leprae of the third passage (mouse-to-mouse), showing a microcapsule (mc), homogeneous bodies (hb), nucleoid (n), mesosomes (ms) and volutin granules (v) (uranyl acetate and lead stain; bar = 0.3 pm).
Beginning from the third passage the M. leprae ultrastructure was stabilized and within eight passages (the observational period) no changes were noted, suggesting "the completion" of the adaptation process. This suggestion is supported by the formation of a microcapsule and the stabilization of the cell wall corresponding to that of the original strain as well as the appearance of volutin granules in the cells from the third and subsequent passages.
The ultrastructure of M. leprae from the first passage to armadillos showed insignificant changes as compared with the original strain. The mode of division is more typical for the original strain than for the strains from the second and the third passages to mice. There are practically no volutin granules in the cytoplasm which also is characteristic for M. leprae strains of the first and second passages.
Thus, our study showed that in the course of intraplantar passage of M. leprae from leprosy patients to mice (Shepard's technique) mycobacterial ultrastructure underwent some changes which were observed within three passages. After having passed through the resting phase (the first passage), M. leprae adapted to a new host by the third passage evidenced by the absence of any differences in ultrastructure of the bacterial cells of the third-to-eighth passages.
The occurrence of volutin granules in mycobacterial cells might be a valuable marker for M. leprae adaptation to a new host. We did not find such inclusions in M. leprae cells of the first and second passages to mice and of the first passage to armadillos.
The appearance of volutin granules or the increase in their amount in bacterial cells might be attributed to the complete adaptation of mycobacteria to their environment (10).
A finding of changeability of M. leprae repeatedly passed to mouse foot pads is supported by the investigations of Hirata and Nakayama who observed bacterial forms morphologically similar to mycobacteria found by us and considered as changeable forms or L-forms of M. leprae (3) .
The possibility of phenotypic changeability of M. leprae strains when passed in vivo must be taken into account in experimental investigations and when screening antileprosy drugs using Shepard's technique.
- Alexander K. Maslov, M. D.
Margarita N. Dyachina, M. D.
Olga V. Kalyanina
Leprosy Research Institute
GSP-7
Astrakhan 414000, Russia
REFERENCES
1. Barkov, A. M. and Peters, M. K. [Selection and genetics of the agents of severe infections.] Anisimov, P. I., ed. Saratov, 1982.
2. Dyachina, M. N., Juscenko, A. A., Ibragimov, C. D., Chernousova, L. N. and Litvinov, V. I. [Identification of antigenic determinants of M. leprae passed on laboratory animals with using monoclonal antibodies.] Zh. Mikrobiol. 1(1994)74-76.
3. Hirata, T. and Nakayama, T. [Cyto-morphological study on M. leprae inoculated and grown in mouse foot-pads.] Lepro 44(1975)163-176.(Eng. abstract)
4. Kolesov, K. A. [On the results of mice inoculated with the material from leprosy patients.] Vestn. Dermatol. Vencrol. 10(1968)55-59.
5. Pervukhin, Y. V., Badowskaya, Z. V., Bronfman, Z. I., Chernysheva, L. M., Nazarov, K. I. and Dyachina, M. N. [A study of some biological properties of mycobacterial strains isolated from leprosy patients and causing a generalized leprosy when passed to mice] In: Clinical Signs, Treatment and Prevention of Leprosy. Juscenko, A. A., ed. Astrakhan, 1984.
6. Pynchuck, L. M., Dyachina, M. N. and Lazovskaya , A. L. [Gas-liquid chromatography of fatty acids of M. leprae passed to laboratory animals.] In: Aktualnye Voprosi Leprologii. Juscenko, A. A., ed. Astrakhan, 1978.
7. Pynchuck, L. M., Dyachina, M. N., Lazovskaya, A. L. and Juscenko, A. A. [High fatty acids in M.leprae isolated from tissues of M. leprae- infected animals.] In: Aktualnye Voprosi Leprologii. Juscenko, A. A., ed. Astrakhan, 1984.
8. Shepard , C. C. The experimental disease that follows the injection of human leprosy bacilli into foot pads of mice. J. Exp. Med. 3(1960)445-454.
9. Vasurenko, Z. P. [Fatty acid spectrum in various strains of Brucella and its relationship to growing millieu.] Zh. Mikrobiol. 8(1977)55-59.
10. Whitehouse, R. L. S., Wong, P. C. and Jackson, F. R. Ultrastructure of Mycobacterium lepraemurium. Int. J. Lepr. 39(1971)151-163.