- Volume 63 , Number 3
- Page: 453–4
Effect of blister calendar packs in enhancing compliance with MDT; the kaduna state (nigeria) experience
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
To the Editor:
Compliance may be described as the willingness to follow or consent to another's wishes, rules or regulation, and thus summarizes a complex behavior pattern. To start with, understanding a health worker's advice is a precondition for complying with it.
To be compliant with multidrug therapy (MDT), a leprosy patient has to: a) attend regularly a clinic to collect the drug for the period between one appointment and the next, and b) ingest the drugs at home according to the prescriptions received at the clinic.
One major development in the delivery of MDT technology is the introduction of blister calendar packs (BCP). Although the attractiveness, social acceptability and ease of accounting of this form of MDT delivery (relative to loose drugs) are not in dispute, at least two major studies on BCP have not shown any significant effect on enhancing compliance relative to loose MDT (3,4).
This report on a comparative study of MDT/BCP and loose MDT relative to compliance by leprosy patients in Kaduna State, Nigeria, demonstrates that BCP may, indeed, enhance compliance with MDT.
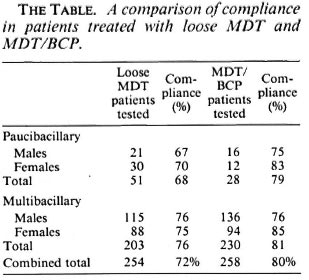
The Kaduna State Tuberculosis and Leprosy (TBL) Programme had a total number of 578 patients with leprosy, 436 of whom were on register for chemotherapy between June 1992 and March 1994 when the studies were conducted. The urine spot test as described by Huikeshoven (2) was used for the study. The first cohort consisted of 254 patients [51 paucibacillary (PB) and 203 multibacillary (MB)] who were on MDT with loose pills as of June 1992. The second cohort consisted of 258 patients (28 PB and 230 MB) on treatment with MDT/BCP.
Each patient's urine was examined on clinic days (without prior notice). Each patient was shown the results of the urine spot test. If positive, the patient was encouraged to carry on with the apparently regular drug intake. In cases with negative spot tests, the patients were interviewed in order to determine the reasons for noncompliance. For the purpose of the study, the patient was described as compliant if tests were positive for dapsone.
The Table shows the results of the comparative study. As can be seen, there was improvement in the compliance with MDT/ BCP compared to loose MDT. Furthermore, when patients on treatment with MDT/BCP were instructed to come to the clinic with empty BCP foils, the percentage of patients testing positive to dapsone by the spot test increased considerably, to 97% (94% males, 100% females) for PB patients and to 95% (95% males, 95% females) for MB patients. It is significant that 8% (5/65) of the patients who tested negative on the spot test claimed that they did not receive the correct amount of loose dapsone on the previous clinic day. Thus, MDT/BCP offers the additional advantage of ensuring that the patient receives the exact quantity of MDT drugs required for treatment.
It has been estimated that MDT presented in BCP adds only US$0.46 and US$0.21 per month for the treatment of MB and PB leprosy, respectively (1). This is a small price to pay for the benefits of compliance and acceptability by patients and the ease with which it can be dispensed to patients, even by general health workers. As the resolve of leprosy control experts that leprosy control be integrated into the general health services becomes realized, one further benefit of MDT/BCP will become more significant, i.e., the diversion (by health workers) of rifampin meant for leprosy patients to (especially) tuberculosis patients. This fear already is being confirmed in at least one program running combined tuberculosis and leprosy services in Nigeria.
- Niyi Awofeso, B.Sc, M.B.Ch.B.
Kaduna State TB and Leprosy Control Services
P.M.B. 1089 Zaria, Nigeria
- Hanneke Lammers
Maricke Verschuuren
Netherlands Leprosy Relief Association
135 Wibautstraat
1097 DN Amsterdam
The Netherlands
Acknowledgment. We are grateful to the Royal Tropical Institute for providing spot test reagents and to the Netherlands Relief Association for their financial support.
REFERENCES
1. Georgiev, G. D. and McDougall, A. C. Blister calendar packs - potential for improvement in the supply and utilization of multiple drug therapy in leprosy control programs. Int. J. Lepr. 56(1988)603-610.
2. Huikeshoven, H. C. J. A Spot Test for Dapsone in Urine; Technical Guide for Testing Patient Compliance with Dapsone Intake in Leprosy. Amsterdam: Netherlands Leprosy Association, 1987.
3. Revankar, C. R., Dhamale, C. B. and Ganapati, R. Experience of multidrug therapy blister calendar packs in urban leprosy control programmes in Bombay. (Letter) Lepr. Rev. 62(1991)336.
4. Revankar, C.R., Gupta, N., Sorensen, B. H., Naik, S. S. and Multicentre Study Group. Further observations on MDT blister-calendar packs in vertical leprosy eradication programmes - a multicentre study (Phase II). Lepr. Rev. 64(1993)250-254.