- Volume 63 , Number 2
- Page: 231–40
Identification of IgM as the leprosy patient serum factor responsible for rapid sedimentation of formolized sheep erythrocytes
ABSTRACT
The serum of some leprosy patients with impaired specific cellular immunity for Mycobacterium leprae causes rapid sedimentation of formolized sheep erythrocytes, a phenomenon known as the Rubino reaction. The Rubino factor was precipitated f rom positive sera by 5% (w/v) polyethylene glycol (PEG), bound to a concanavalin A (ConA)-Sepharose column and cluted with D-mannose, and was also eluted f rom a Mono Q column, pH 8.0, with 0.4 M NaCl. The Rubino factor was eluted in a volume which coincided with that of human serum IgM f rom a Sepharose 6 column. IgM was present in the preparation obtained by this sequence of chromatographic procedures. The correspondence of IgM with the Rubino factor was demonstrated by the following data: a) the Rubino factor was adsorbed to rabbit IgG antihuman IgM-agarose and the activity was recovered in the acid eluate of the column; b) the Rubino reaction was inhibited in the presence of rabbit antihuman IgM antibodies. This behavior was not observed when the same procedures were carried out using anti-α2-macroglobulin antibodies as a control. The rapid sedimentation of formolized sheep red cells caused by the serum of lepromatous leprosy patients was not inhibited by phenolic glycolipid-I, suggesting that the IgM responsible for the Rubino reaction is not directed to this antigen which is specific for M. leprae. There was no correlation between the positivity of the Rubino reaction and the increase in total serum IgM levels observed in 42% of the lepromatous patients evaluated. The demonstration that the Rubino factor is an IgM now permits the identification of the epitope recognized by it, and this may be used as a tool to understand the spécifie cellular immune unresponsiveness which characterizes lepromatous leprosy.RÉSUMÉ
Le serum de certains malades de la lèpre présentant une immunité cellulaire spécifique vis-à-vis du Mycobacterium leprae endommagée provoque une sédimentation rapide des globules rouges formolisés de mouton, un phénomène connu sous le nom de réaction de Rubino. Le facteur de Rubino a été précipité à partir de serum positif par du polyéthylène glycol (PEG) à 5% (poids/vol.), lié à une colonne de concanavalinc A (Con A)-Sepharose et dissout avec du D-mannose, et a aussi été dissout d'une colonne de Mono Q, au pH 8.0, avec du NaCl 0.4 M. Le facteur de Rubino a été dissout dans un volume coïncidant à celui des IgM sériques humains à partir d'une colonne de sepharose 6. Des IgM étaient présents dans la préparation obtenue par cette séquence de procédures chromatographiques. La correspondance des IgM avec le facteur de Rubino a été démontrée par les données suivantes: a) le facteur de Rubino était adsorbe au complexe IgG de lapin anti-IgM humain-agarosc et l'activité a été récupérée dans I'éluat acide de la colonne; b) la réaction de Rubino était inhibée en présence d'anticoprs de lapins anti-IgM humains. Ce comportement n'était pas observé quand les mêmes procédures étaient exécutées en utilisant des anticorps anti-α,-macroglobulines comme témoins. La sédimentation rapide des globules rouges formolisés de mouton par le serum de patients lépromateux n'était pas inhibée par le glycolipide phenoliquc-I, ce qui suggère que les IgM responsables de la réaction de Rubino ne sont pas dirigés vers cet antigène spécifique de M. leprae. Il n'y avait pas de correlation entre la positivité de la réaction de Rubino et l'augmentation des taux d'IgM dans le scrum total observée chez 42% des patients lépromateux évalués. La démonstration que le facteur de Rubino est une IgM permet à présent l'identification de l'épitope recconu par celui-ci, et ceci pourrait être un moyen pour comprendre la nonréactivité de l'immunité cellulaire spécifique qui caractérise la lèpre lépromateuse.RESUMEN
El suero de algunos pacientes con lepra y con alteración en su inmunidad celular específica hacia Mycobacterium leprae, acelera la sedimentación de eritrocitos de carnero tratados con formol, un fenómeno conocido como reacción de Rubino. El factor Rubino de un suero positivo se precipitó con polietilén glicol (PEG) al 5% (p/v), se adsorbió a una columna de sefarosa-concanavalina A, y se eluyó con D-manosa. También se adsorbió a una columna Mono Q, pH 8.0, y se eluyó con NaCl 0.4 M. El factor Rubino se eluyó de una columna de sefarosa 6 en un volumen que coincide con el de la IgM del suero. La IgM estuvo presente en la preparación obtenida siguiendo esta secuencia de procedimientos cromatográlicos. La correspondencia de la IgM con el factor Rubino fue demostrada por los siguientes datos: (a) el factor Rubino fue adsorbido a columnas de agarosa con IgG anti-IgM humana y la actividad se recuperó en el cluido ácido de la columna; (b) la reacción de Rubino se inhibió en presencia de anticuerpos de conejo anti-IgM humana. Los anticuerpos anti-alfa 2 macroglobulina no inhibieron la reacción de Rubino. El glicolípido fenólico I (PGL-I) tampoco inhibió la reacción de Rubino; esto sugiere que la IgM responsable de la reacción de Rubino no está dirigida contra este antígeno específico de M. leprae No hubo correlación entre la positividad de la reacción de Rubino y el incremento en los niveles totales de IgM observado en el suero del 42% de los pacientes lepromatosos evaluados. La demostración de que el factor Rubino es una IgM permite ahora la identificación del epítopo que reconoce, y esto puede usarse como una herramienta para entender la anergia celular específica que caracteriza a la lepra lepromatosa.A reaction described by Rubino (23), which consists of the rapid sedimentation of formolized sheep red cells incubated with serum, is positive in some leprosy patients. The sensitivity of the Rubino reaction is low and variable but its specificity for leprosy is absolute (8). The Rubino reaction is due to a specific factor present in the serum of some leprosy patients plus a nonspecific component present even in the serum of normal individuals (17). The specific factor responsible for the Rubino reaction is selectively adsorbed by formolized red cells, but differs from those heteroagglutinins that react with both natural and formolized red cells (24,25). The Rubino factor is active after heating at 56ºC for 60 min (17). Red cells sensitized with the Rubino factor consume complement (26).
The association between the Rubino reactivity and plasma gamma-globulins was suggested but not demonstrated by de Almeida (6) in a review of the serology of leprosy. He pointed out that there was no correspondence between the Rubino factor and the complement-fixing antibodies that recognize tubercle antigen preparations. Later he reported the inhibition of the Rubino reaction when it was carried out in the presence of some preparations of antigens from Mycobacterium leprae, M. tuberculosis or some atypical mycobacteria (7,8). Garcia Lima and Laure (10) reported that they had purified the Rubino factor, which had a molecular mass of 76 kDa and pi of 5.7, but did not provide supporting data.
Patients with lepromatous leprosy, the polar form of the disease characterized by the absence of the protective immune response to M. leprae, present the highest frequency of positivity to the Rubino reaction (55%) (9). This association motivated our interest in the identification of the Rubino factor described in the present report.
MATERIALS AND METHODS
Human sera. Sera from leprosy patients were obtained from the Teaching Health Center of the Department of Social Medicine and from the Outpatient Clinic of the Discipline of Dermatology, Department of Internal Medicine, Faculty of Medicine of Ribeirão Preto, University of São Paulo (FMRP-USP). All sera were held for 30 min at 56ºC to inactivate complement. Each serum sample was submitted to the Rubino reaction: those that reacted positively and had no agglutinins for natural sheep red blood cells (SRBC) were combined, and this pool was used as the source of the "Rubino factor." A pool of normal human sera (NHS) from healthy volunteers (students of FMRP-USP) was used as the negative control after each serum was shown to be negative for the Rubino reaction. IgM levels in serum samples from lepromatous leprosy patients (20 Rubino positive and 20 Rubino negative) and from negative controls (5 NHS) were determined by a nephelometric method (Beckman Instruments, Fullerton, California, U.S.A.). The results obtained for the various groups were compared by the Student t test (p < 0.05) and by the nonparametric Wilcoxon test.
Sheep red blood cells. SRBC were obtained from healthy sheep maintained in the Central Animal House, University of São Paulo, Ribeirão Preto Campus. Erythrocytes were separated by centrifugation at 280 X g from blood collected into Alsever solution, washed three times with 0.85% (w/v) saline, and treated with 10% (v/v) formaldehyde for 48 hr at room temperature. In some experiments SRBC were treated with similar concentrations of glutaraldehyde or acetaldehyde. SRBC were washed with phosphate buffered saline (PBS) resuspended at a concentration of 3.5 to 4.0 x 106 SRBC/ml in PBS (24,25).
Rubino reaction. The Rubino reaction consists of incubating serum (final dilution 1/10) with formolized SRBC (7 to 8 X 105 cells per tube) in a final volume of 1 ml (PBS diluent) for 1 hr at 37ºC. The reaction is considered to be positive when a clear supernatant and sedimented formolized erythrocytes are obtained, while the formolized SRBC used in the negative controls with NHS continue to be in suspension (24.25) yjje same assay was used to test the Rubino activity in fractions prepared from serum by precipitation and by subsequent chromatographic procedures. In these assays NHS was used to supplement the reaction medium at a final dilution of 1/10. The negative controls were similar fractions obtained from NHS in parallel by the same procedures.
We have not been able to quantitate Rubino factor activity because the response, i.e., the rapid sedimentation of formolized SRBC, is either positive or negative and not graded.
Antisera. The following antisera and conjugates were utilized: rabbit serum antihuman IgG γ chain, rabbit serum anti-human IgA α chain, rabbit serum anti-human IgM μ chain (Behringwerke AG, Marburg, Germany); rabbit serum anti-human α2macroglobulin (α2M) (Dako A/S, Glostrup, Denmark); monoclonal antibody (mouse IgGt) anti-65-kDa antigen (heat shock protein) from M. leprae (kindly provided by Prof. Celio Lopes Silva, FMRP-USP); rabbit serum anti-human albumin (kindly provided by Prof. Terezila Machado Coimbra, FMRP-USP); goat IgG anti-human IgM µ chain peroxidase conjugate (Sigma Chemical Company, St. Louis, Missouri, U.S.A.); sheep IgG anti-rabbit IgG peroxidase conjugate, and rabbit IgG anti-mouse IgG peroxidase conjugate (11).
Polyethylene glycol (PEG) precipitation of serum proteins. The pool of Rubino-positive sera was submitted to successive precipitation with polyethylene glycol 4000 in the concentration ranges of 0%-5%, 5%12% and 12%-20% (11). At each step, the material was maintained under constant shaking overnight at 4ºC and centrifuged at 10,000 x g for 10 min. A sample of each precipitate was resuspended in a volume of PBS corresponding to the initial sample volume, dialyzed against PBS, supplemented with NHS (1/10), and submitted to the Rubino reaction. The 5% PEG fraction contained the Rubino-positive activity and was divided into aliquots and stored at - 70ºC.
Rubino reaction activity after enzymatic digestion. The 5% PEG precipitate (600 ng protein) obtained from 0.1 ml of the pools of Rubino-positive sera or NHS (negative control) was incubated in 0.6 ml of PBS with or without pronase (0.6 μg) at 20ºC for 24 hr. The incubates were supplemented with NHS (1/10) and tested for Rubino activity as described above.
Rubino reaction activity in presence of EDTA. The 5% PEG precipitate (600 Mg protein) obtained from 0.1 ml of the pools of Rubino-positive sera or NHS (negative control) was incubated in 0.6 ml of PBS with or without 10 mM EDTA at 20ºC for 1 hr. The incubates were supplemented with NHS (1/10) and tested for Rubino activity as described above.
Affinity chromatography. Samples obtained by precipitation of 2.0 ml of serum with 5% PEG (12 mg protein) were submitted to affinity chromatography on each of six different columns: D-galactose-agarose (Pierce Chemical Company, Rockford, Illinois, U.S.A.); D-mannose-agarose (Pierce Chemical); guar gum (p-galactomannan, kindly prepared and provided by Prof. Renato Moreira, Federal University of Ceara); protein A-agarose (Pharmacia LKB Biotechnology, Uppsala, Sweden); jacalin-agarose (prepared in our laboratory and kindly provided by Prof. Laura Maria de Vasconcelos), and concanavalin A-agarose (ConA-Sepharose; Pharmacia LKB). ConA-Sepharose was submitted to crosslinking with glutaraldehyde in the presence of D-mannose (28). The columns were equilibrated and developed with 10 mM PBS and 0.5 M NaCl, pH 7.2. The material bound to each of the columns was eluted with 0.1 M galactose (D-galactose-agarose); 0.1 M D-mannose (D-mannose-agarose); sequentially with 0.1 M D-galactose and 0.1 M glycine buffer-HC1, pH 2.5 (guar gum); 0.1 M glycine-HCl buffer, pH 2.5 (protein A-agarose); 0.1 M D-galactose (jacalin-agarose); 0.1 M D-mannose (ConA-Sepharose). The material bound or not bound to each column was tested for Rubino reaction activity.
Ion-exchange chromatography of ConA-Sepharose bound active material. The material eluted by D-mannose from the ConA-Sepharose resin was dialyzed and resuspended in buffer A (20 raM Tris-HCl, pH 8.0) and submitted to anion exchange chromatography (12) on a Mono Q HR 5/5 column (Pharmacia LKB) equilibrated with buffer A at room temperature and at a flow rate of 1 ml/min. A linear gradient from 0 to 1 M NaCl (in 20 ml) was used to elute the material retained by the column, and 1 ml fractions were collected.
Gel filtration chromatography. The fraction containing Rubino activity after anion exchange chromatography was dialyzed against 10 mM PBS and 150 raM NaCl, pH 7.2, and submitted to gel filtration on Sepharose 6 HR 10/30 (Pharmacia LKB). The column was equilibrated and eluted with the sample buffer at a flow rate of 0.1 ml/min, and 1-ml fractions were collected. The column was calibrated (1) with Blue dextran 2000 (BD, approximately 2000 kDa); purified IgM, 950 kDa (Sigma); thyroglobulin, 669 kDa; ferritin, 440 kDa; aldolase, 158 kDa; bovine serum albumin (BSA), 67-kDA (Pharmacia LKB).
Adsorption of the 5% PEG precipitable Rubino factor to immobilized antibodies. Sepharose activated with cyanogen bromide (1 ml each) (Pharmacia LKB) was reacted with rabbit IgG anti-human IgM, or with rabbit IgG anti-human α2M (both rabbit IgG were purified on an immobilized protein A column). The 5% PEG precipitates (12 mg protein) from 2 ml of the pools of Rubinopositive sera or NHS were passed through columns of the immobilized antibodies. The nonadsorbed material was recovered by washing with PBS. The bound material was eluted with 0.1 M glycine-HCl buffer, pH 2.5, and neutralized with 2 M Tris-HCl, pH 8.0, immediately after collection. Bound and unbound materials obtained by each procedure were tested for Rubino-reaction activity. Samples of bound and unbound materials to the immobilized rabbit IgG antihuman IgM were also subjected to SDS-PAGE and to immunoblotting using antihuman IgM or anti-human α2-M antibodies.
Inhibition assay of serum Rubino factor. The 5% PEG precipitate (600 /μg protein) from 0.1 ml of the pool of Rubino-positive sera and formolized SRBC (7 to 8 X 105 cells per tube) were incubated in PBS at a final volume of 0.6 ml in the presence of human IgM (Sigma), semisynthetic natural disaccharide (epitope of phenolic glycolipid-I; PGL-I) conjugated with BSA preparation (ND-O-BSA; kindly provided by Prof. Celio Lopes Silva, FMRP-USP), and rabbit IgG anti-human IgM, rabbit IgG anti-human IgG, rabbit IgG anti-human IgA or rabbit IgG anti-α2M for 1 hr at 37ºC. The incubates were supplemented with NHS (1/10) and tested for Rubino activity. The Rubino reaction was interpreted as described above.
Inhibition assay of Rubino factor adsorbed to sensitized SRBC. The 5% PEG precipitate (3 mg protein) from 0.5 ml of the pool of Rubino-positive sera was incubated with formolized SRBC (7 to 8 X 105 cells per tube) for 1 hr at 37ºC. After incubation, the supernatant was tested for Rubino reaction activity. A positive response indicated that the Rubino factor used to sensitize the SRBC was in excess. The sensitized SRBC were washed three times with PBS, resuspended in the same buffer, and incubated with the substances described in the previous section. After 1 hr at 37ºC the incubate was supplemented with NHS (1/10) or with PBS (negative control). The Rubino reaction activity was tested as described above.
Electrophoresis and immunoblotting. Electrophoretic analysis was performed with 7% or 10% polyacrylamide gel under dissociating conditions (SDS-PAGE) using the Mini V-8.10 vertical gel electrophoresis system (GIBCO BRL, Gaithersburg, Maryland, U.S.A.) or Phast gel gradient media (8%-25%) in the Phast system (Pharmacia LKB) (16). The samples were resuspended in 0.5 M Tris-HCl buffer, pH 6.5, and 2% SDS and, in some cases, 3.5% mercaptoefhanol was added. Electrophoresis was carried out for 30 min (80-120 mA, 200 V) in the BRL system and for 20 min in the Phast system (10 mA, 100-250 V). The gels were stained with PhastGel Blue R (Pharmacia LKB) or with silver (3).
In some experiments the gels were blotted onto nitrocellulose membranes using the Mini V-8.10 vertical gel electrophoresis system or the Phast system. When the Mini V-8.10 system was used, electrotransfer was carried out in a cuvette containing 20 mM Tris-glycine, pH 8.3 (85-135 mA, 150 V) for 1.5 hr. When the Phast system was used, electrotransfer was carried out in a semihumid transfer chamber (25 mA, 0-20 V) for 15 min. Each transferred sample was incubated with one of the following primary antibodies: 1) rabbit IgG anti-human IgM H chain, 2) rabbit IgG anti-human α2M serum, 3) rabbit IgG anti-human IgG γ chain, 4) rabbit anti-human albumin serum, and 5) mouse monoclonal IgG1 anti-65 kDa M. leprae antigen. The membrane was then incubated with anti-rabbit or anti-mouse IgG peroxidase conjugates. In some experiments, the membrane was developed directly with goat IgG anti-human IgG γ chain or with goat IgG anti-human IgM μ chain peroxidase conjugates. The antigen-antibody reactions were detected with diaminobenzidine (DAB) (Sigma) in the presence of 30% hydrogen peroxide (Sigma).
RESULTS
General properties of Rubino factor activity. Rubino factor activity was recovered only in 5% (w/v) PEG precipitate of the pool of sera of patients positive for the Rubino reaction. This fraction, which corresponds to 10% of the total scrum protein (6 mg/ml serum protein), was used in the studies reported here.
The Rubino reaction activity in the 5% PEG fraction was inactivated by incubation with pronase (E/S = 0.1%, w/w) for 24 hr at room temperature in PBS, pH 7.2. Incubation of the 5% PEG fraction with 100 mM EDTA for 1 hr at room temperature in PBS, pH 7.2, had no effect on the Rubino reaction activity, indicating that neither Ca 2 + or Mg 2 + were required for activity. This would have been the case if activity had been due to C-reactive protein the levels of which are increased in erythema nodosum leprosum that occurs in about half of the patients with lepromatous leprosy (9).
Since formolization of the SRBC would be expected to both block amino groups and create crosslinks, we tested acctaldehyde and glutaraldehyde for their ability to sensitize SRBC. Glutaraldehyde-treated, but not acetaldehyde-treated SRBC gave a positive Rubino reaction, suggesting that the surface membrane proteins need to be crosslinked to support the Rubino reaction.
Chromatographic properties of the Rubino factor. Rubino factor activity present in the 5% PEG precipitate was adsorbed to a column of immobilized ConA but not to the columns of the immobilized sugars D -galactose, D -mannose or D -galactomannan (guar gum), immobilized protein A or immobilized jacalin. Figure la shows the elution of Rubino factor activity from ConA-sepharose by D-mannose. Rubino factor activity adsorbed to ConA was shown to be anionic and partially purified by chromatography on Mono Q eluted with a linear gradient of NaCl (Fig. 1b). The apparent molecular mass of the factor responsible for Rubino activity after purification with ConA and Mono Q was estimated to be 950 kDa on the basis of its clution position from a Sepharose 6 gel filtration column, which coincided with that of IgM (Fig. 1c). The presence of guanidine-HCl in the buffer did not modify the apparent molecular weight of the Rubino factor (data not shown), suggesting that the high molecular weight was not due to polymerization of the factor or to its association with other serum or parasite proteins.
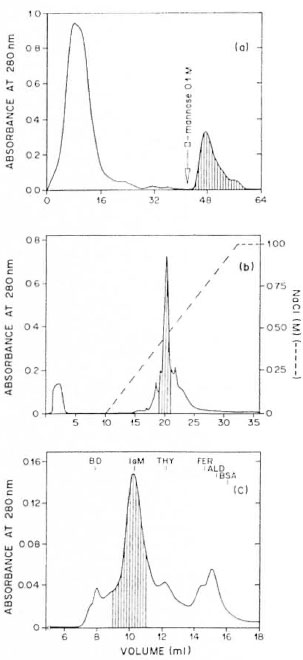
Fig. 1. Chromatographic properties of the Rubinofactor. a) = Chromatography of the 5% PEG precipitate(12 mg) of the pool of Rubino-positive sera. The column (bed volume 8 ml) of immobilized ConA wasequilibrated with 10 mM PBS and 0.5 NI NaCI, pH7.2, at 4°C. After sample application, 42 ml of the samebuffer was used to remove unbound material. Boundmaterial was eluted with 20 ml 0.1 NI D-mannose inPBS. Two ml fractions were collected. The recovery of protein was 86%. The fraction eluted with D-mannose corresponded to 20% of the recovered material. Thehatched region indicates elution position of Rubinoactivity. b) = Ion-exchange chromatography of theConA-bound Rubino-positive material (4.5 mg fromthree ConA columns). The column, Mono Q HR 5/5,was equilibrated and initially eluted with buffer A (20mM Tris-HCI pH 8.0) at a flow rate of I ml/min at room temperature. Retarded material was eluted using a 0 to 1 M NaCl linear gradient. One hundred μl of each 1 ml fraction was supplemented with normal human serum and submitted to the Rubino reaction. The hatched region indicates fractions containing Rubino reaction-positive activity: 30% of applied protein was eluted in the Rubino reaction-positive effluent, c) = Gel filtration on sepharose 6 HR 10/30 of the Rubino reaction-positive material (1 mg) obtained by anionexchange chromatography of the ConA-bound fraction. The column was equilibrated and eluted with PBS 10 mM NaCl 0.5 M, pH 7.2, at a flow of 0.1 ml/min at room temperature. The effluent was monitored by absorbance at 280 nm. One hundred of μl each fraction (1 ml) was supplemented with normal human scrum and submitted to the Rubino reaction. The hatched region indicates the fractions containing positive Rubino activity (200 μg protein). The column was calibrated with markers of known molecular mass: BD, blue dextran (approximately 2000 kDa); IgM, immunoglobulin M (950 kDa); THY, thyroglobulin (669 kDa); FER, ferritin (440 kDa); ALD, aldolase (158 kDa); BSA, bovine serum albumin (67 kDa), whose elution volumes are indicated.
Identification of IgM as serum factor responsible for Rubino reaction activity.' The data, which indicate that the Rubino factor behaves like IgM under different chromatographic conditions, do not eliminate other possibilities, such as α2M. When the preparation obtained by gel filtration was submitted to 7% SDS-PAGE (in the absence of mercaptoethanol) only a material of high molecular weight was observed on the top of the gel (data not shown). Electrophoresis of the same samples (10% SDS-PAGE) in the presence of mercaptoethanol revealed a major band with a molecular mass of 70 kDa and much weaker bands at 65, 55 and 25 kDa (Fig. 2a, lane 1). When the gel was blotted onto nitrocellulose only the 70-kDa band reacted with anti-human IgM conjugate (Fig. 2a, lane 2). Neither anti-human 2-macrobulin, anti-human albumin, nor anti-65-kDa antigen from M. leprae reacted with the blotted nitrocellulose. These antigens, if present, would have generated at least one of the fragments observed. We have characterized the 65-, 55- and 25-kDa bands only in negative terms because they did not react with the antibodies tested.

Fig. 2. SDS-PAGE and immunoblotting. a) Purified Rubino reaction-positive preparation after gel filtration (Fig. 1c); 10% Mini V 8.10 gel was used under reducing conditions. Lane 1 = SDS-PAGE, silverstained gel; lane 2 = immunoblotting developed with anti-human IgM conjugate, B) Anti-human IgM adsorbed and nonadsorbed materials from Rubino-positive 5% PEG precipitate; 8% to 25% Phast gel gradient media was used under reducing conditions; lane 3 = immunoblot of nonadsorbed material developed with anti-human IgM conjugate; lane 4 = immunoblot of the adsorbed material developed with anti-human IgM conjugate. The electrophoretical mobility of proteins with known MW (kDa) is indicated.
These data suggested that the Rubino factor was indeed an IgM. We tested this idea by assaying the adsorption of Rubino factor activity to immobilized anti-IgM or antia 2M antibodies. The activity was recovered in the material adsorbed to the anti-human IgM column and in the material not adsorbed to anti-human a 2M column (Table 1), showing that the Rubino factor behaved like IgM.
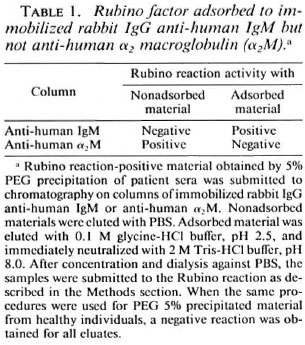
The adsorbed and nonadsorbed materials obtained by chromatography on the antihuman IgM column were submitted to electrophoresis under reducing conditions followed by immunoblotting to nitrocellulose; no bands were developed with anti-μ chain antibodies in the nonadsorbed material (Fig. 2b, lane 3); whereas a 70-kDa band was recognized by the anti-μ chain in the adsorbed material which had Rubino factor activity (Fig. 2b, lane 4). Anti-human α2M antibodies recognized only bands present in the nonadsorbed material (data not shown) which did not have biological activity.
The Rubino reaction was inhibited when positive sera and formolized SRBC were incubated in the presence of rabbit anti-human IgM antibodies (Table 2); whereas no inhibition occurred in the presence of antibodies with other specificities, including anti-human α2M. IgM obtained from normal scrum did not compete with the Rubino factor in the development of the reaction. Similar results were obtained when the same antibodies or IgM were placed in contact with the Rubino factor bound to formolized SRBC. Only anti-IgM antibodies inhibited the sedimentation triggered by supplementation of the medium with normal serum. In order to determine if the IgM corresponding to the Rubino factor was specific for M. leprae PGL-I antigen, we attempted to inhibit the Rubino reaction with ND-O-BSA, which contains the PGL-I sugar epitopes. No inhibition of the Rubino reaction was obtained with lOμg/ml of the ND-O-BSA.
Measurement of serum IgM in serum tested. We determined the serum levels of IgM in 40 sera from lepromatous leprosy patients (20 Rubino-positive and 20 Rubinonegative sera) and in five sera from normal subjects. The data presented in Figure 3 show that 50% of the Rubino-negative lepromatous leprosy sera and 35% of the Rubino-positive sera had higher than normal IgM levels. No statistically significant difference in serum IgM levels was detected between Rubino-positive and Rubino-negative individuals.
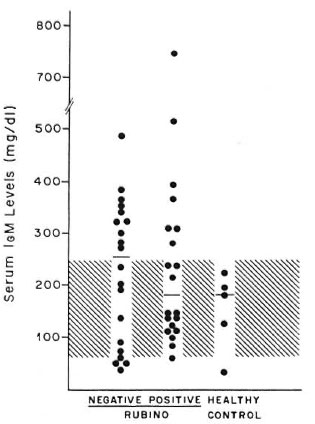
Fig. 3. The presence of Rubino factor is not associated with modified IgM levels. Scrum IgM levels (●) in two groups of lepromatous leprosy patients (40 sera = 20 Rubino reaction-negatives and 20 Rubino reaction-positives) and in healthy individuals (5 sera). The hatched region indicates the normal range; horizontal lines = median for each group.
DISCUSSION
In the present paper we provide evidence that the factor in the sera of patients with lepromatous leprosy responsible for the Rubino reaction activity is an immunoglobulin M. However, the present data do not permit us to distinguish between the presence of IgM and IgM-antigen complex in patient serum. The paucity of studies about the Rubino reaction initially left many possibilities open about the nature of the substance(s) responsible for the phenomenon, with the impossibility of inferring even if the factor was a host or a parasite component. In the separation procedures carried out here, we first noted recovery of the activity in the 5% PEG precipitate, in the material retarded in the ConA-Sepharose column and eluted with D-mannose, in the material delayed in a Mono Q column and eluted in the salt gradient, and in the elution volume of 950 kDa proteins by gel filtration (Fig. 1). These characteristics of the Rubino factor were retrospectively identified as coinciding with the properties of human IgM. IgM and IgM-antigen complexes are insoluble in 10% PEG (11,22); IgM interacts with ConA (32), and is retarded on anion-exchange column (27). A second protein, α2 macroglobulin, shares some of these properties (30,31), but this possibility was eliminated by the fact that the column with immobilized anti-α2M antibodies did not adsorb the Rubino reaction activity; whereas the anti-IgM antibody column did (Table 1).
The fact that the Rubino factor activity was adsorbed to immobilized anti-human IgM antibodies and recovered in the acid eluate provides strong evidence that the Rubino factor corresponds to IgM. The second strong line of evidence was provided by the inhibition of the Rubino reaction by the addition of anti-human IgM to the reaction medium. Marchoux and Caro (17) observed that formolized SRBC obtained from a Rubino-positive reaction, even after washing, continued to sediment rapidly when placed in medium containing normal human serum, and they proposed that the reaction is determined by a specific factor in the serum of certain leprosy patients which selectively binds to formolized SRBC and by a nonspecific factor present in all normal sera. We confirmed the requirement for normal sera factor and also used this observation to design an inhibition assay in which rabbit antibodies of different specificities could be tested to determine if they inhibit rapid sedimentation of sensitized SRBC. The inhibition occurred only when rabbit anti-human IgM antibodies were added to the reaction medium, and not when rabbit anti-human α2M, anti-human IgG or anti-human IgA antibodies were used. Similar results were obtained when rabbit antibodies were added to patient serum before contact with formolized SRBC (Table 2).
The correspondence of the Rubino factor of IgM led us to determine IgM levels in patients' sera. We did not find a correlation between Rubino reaction positivity and IgM levels, confirming the results of Arruda, et al. (2) and contradicting those reported by Silva, et al. (29)
Now that we have shown that the Rubino factor is an IgM, one would like to know to which antigen the Rubino factor IgM is directed. The most likely candidate is a mycobacterial antigen, but the possibility that it is an autoantigen cannot be excluded because leprosy is known to have an autoimmune component (21). The Rubino factor IgM described docs crossreact with a structure on the surface of formolized SRBC. The modifications of SRBC after treatment with formaldehyde would be expected to be alkylation and crosslinking of membrane glycolipids and proteins via amino groups (18). Crosslinking was shown to be essential for the Rubino reaction because formaldehydeor glutaraldehyde-treated erythrocytes, but not acctaldehyde-treated erythrocytes or untreated erythrocytes, sedimented rapidly. An observation not documented in the present report suggests that the Rubino factor may be specific for a carbohydrate antigen since the adsorbed Rubino factor activity was eluted from the surface of formolized SRBC by 0.1 M borate buffer, pH 6.5 (15). Correspondence between the antigen recognized by the Rubino factor and the PGL-I antigen of M. leprae is suggested by the following data: a) the carbohydrate portion of PGL-I is the antigenic determinant of the molecule (4,34); b) PGL-I is a unique antigen of M. leprae (13,14); c) more than 80% of the antibodies that recognize PGL-I are IgM (35). However, the Rubino reaction was not inhibited by ND-O-BSA at a final concentration of 10 μg/ml, which would have been expected if the PGL-I sugar was the epitope recognized by the Rubino factor (Table 2).
The Rubino factor may be an autoantibody. Phenomena of autoimmunity in leprosy may be due to the host response to the stress proteins of M. leprae, which are highly conserved in the evolutionary scale, leading to the recognition of self-structures (36). Polyclonal activation of B lymphocytes, another phenomenon observed in leprosy (20), may also lead to the formation of autoantibodies, among them the IgM corresponding to the Rubino factor.
The correspondence of the Rubino factor to serum IgM is clearly demonstrated here. Additional studies are needed to determine the specificity of these antibodies which should open new perspectives in the understanding of the changes in the immune response which occur in patients with lepromatous leprosy.
Acknowledgment. This work was supported by FA-PESP, FINEP, FAEPA and CNPq. Ademilson Panunto-Castelo received a master's research fellowship from FAPESP. We thank the professionals at Centro de Saúde Escola of the FMRP-USP for valuable cooperation and Mrs. Sandra Maria Oliveira Thomaz and Mrs. Imaculada Conceição Bragheto for the technical assistence. We also thank Dr. Norma Tiraboschi Foss for many useful discussions. We are grateful to Dr. Lewis Joel Greene for the critical review and discussion of the manuscript.
REFERENCES
1. ANDREWS, J. The gel filtration over a wide range. J. Biochem. 96(1965)595-606.
2. ARRUDA, M. S., COSTA, H. C, SOUZA, L. C. D. and NOBRE, L. A. S. Avaliação das immunoglobulinas séricas cm pacientes com hanscniasc virchoviana. Salusvita 6(1987)96-101.
3. BLUM, H., BEIER, H. and GROSS, H. J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8(1987)93-99.
4. CHO, S. N., YANAGIHARA, D. L., HUNTER, S. W.,GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use serodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
5. CURBAN, G. V. Contribuição para o estudo da reação de Rubino. Rev. Bras. Leprol. 30(1962)179-216.
6. DE ALMEIDA, J. O. Serology in leprosy. Bull. WHO 42(1970)673-702.
7. DE ALMEIDA, J. O. and KWAPINSKI, J. B. Reactividade de antígenos de actinomicetos com soros de lepra, avaliada por imunofluorescência cm suporte de acetato de celulose. Publçõcs. Cent. Estud. Lepr. 14(1974)73-89.
8. D E ALMEIDA, J. O. Inhibition of Rubino factor as a test for detecting antigens common to leprosy bacilli. (Letter) Int. J. Lepr. 46(1978)436.
9. Foss, N. T., OLIVEIRA, E. B. and SILVA, C. L. Correlation between TNF production, increase of plasma C-reactive protein level and supression of T lymphocyte response to concanavalin A during erythema nodusum leprosum. Int. J. Lepr. 61(1993)218-226.
10. GARCIA-LIMA, E. and LAURE, C. J. Isolation and identification of a substance from serum of leprosy patients. (Letter) Int. J. Lepr. 58(1990)726.
11. HAO, Y. L., INGHAM, K. C. and WICKERHAUSER, M. Fractionation precipitation of proteins with polyethylene glycol. In: Methods of Plasma Protein Fractionation. Curling, I. M., cd. London: Academic Press Inc., 1980, pp. 57-74.
12. HIMMELHOCH, S. R. Chromatography of proteins on ion-exchange adsorbents. Methods Enzymol. 22(1971)273-286.
13. HUNTER, S. W. and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
14. HUNTER, S. W., FUJIWARA, T. and BRENNAN, P. J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 257(1982)10557-10578.
15. KARAT, E. V. and MAYER, M. M. Experimental Immunochemistry. 2nd edn. Springfield: Charles C. Thomas Publisher, 1961.
16. LAEMMLI, U. K. Clevage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1970)660-685.
17. MARCHOUX, E. and CARO, J. Méthode de diagnostic sérologique de la lèpre. Ann. Inst. Pasteur 42(1928)542-552.
18. MEHTA, N. G. ABO (H) blood group antigens of the human erythrocytes membrane: contribution of glycoprotein and glycolipid. J. Membr. Biol. 52(1980)17-24.
19. OPROMOLLA, D. V. A., ARRUDA, M. S., URA, S., PERNAMBUCO, J. C, BASTAZINI, I. and FLEURY, R. N. Aspectos evolutivos da reação de Rubino. Med. Cutan. Ibero Lat. Am. 10(1982)9-14.
20. RAMOS, T., ZALCBERG-QUINTANA, I., APPELBERG, R., SARNO, E. N. and SILVA, M. T. T-helper cell subpopulation and the immune spectrum of leprosy. Int. J. Lepr. 57(1989)73-81.
21. RAWLINSON, W. D., BASTEN, A. and HARGRAVE, J. C. Clinical significance of changes in scrum proteins, immunoglobulins, and autoantibodies in leprosy. Int. J. Lepr. 55(1987)277-285.
22. ROBINSON, M. W., SCOTT, D. G. I., BACON, P. A., WALTON, K. W., COPPOCK, J. S. and SCOTT, D. L. What proteins are present in polyethylene glicol precipitates from rheumatic sera? Ann. Rheum. Dis. 48(1989)496-501.
23. RUBINO, M. C. Nueva reacción sorológica en la lepra. Rev. Méd. Uruguay 29(1926)143-155.
24. RUBINO, M. C. Nouvelle réaction sorologiquc dans la lèpre. Rev. Méd. Uruguay 32(1929)85-116.
25. RUBINO, M. C. Séro-diagnostic de la lèpre par l'agglutino-sédimentation des globules de mouton formóles. Ann. Inst. Pasteur 47(1931)147-172.
26. RUBINO, M. C. Untersuchungen zur Verwcndung formolfixicrter Hammelblutkoerperchen in der Scrodiagnostik. Zentralbl. Bakteriol. 120(1931)378-384.
27. SAMPSON, I. A., HODGEN, A. N. and ARTHUR, I. H. The separation of immunoglobulin M from human serum by fast protein liquid chromatography. J. Immunol. Methods 69(1984)9-15.
28. SCHER, M. G.,RESNECK, W. G. and BLOCK, J. Stabilization of immobilized lectin columns by crosslinking with glutaraldehyde. Anal. Biochem. 177(1989)168-171.
29. SILVA, O. P., FERRI, R. G., MORAES, N. and MARQUES, A. L. V. Estudos imunoquímicos na hanseníasc. II -Quantificação de imunoglobulinas séricas. Tentativa de associação com a reação de Rubino. Hansenol. Int. 1(1976)43-51.
30. SILVESTRINI, B., GUGLIELMOTTI, A., SASO, L. and CHENG, C. Y. Changes in concanavalin A-reactive proteins in inflammatory disorders. Clin. Chem. 35(1989)2207-2211.
31. STAHLER, M. S., GUGLIELMOTTI, A., MATHUR, P. P., SILVESTRINI, B., BARDIN, C. W. and CHENG, C. Y. CM B-18 is a new Sertori cell secretory protein for studying testicular physiology. J. Cell. Biol. 107(1988)630a (abstract no. 3572).
32. WEINSTEIN, Y., GIVOL, D. and STRAUSBAUCH, P. H. The fractionation of immunoglobulins with insolubilized concanavalina A. J. Immunol. 109(1972)1402-1404.
33. WILSON, M. B. and NAKANE, P. K. Immunofluorescence and Related Techniques. Amsterdam: Elscvicrs/North-Holland, 1978.
34. YOUNG, D.B. and BUCHANAN, T. M. A serological test for leprosy with a glicolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
35. YOUNG, D. B., DISSANAYAKE, S., MILLER, R. A., KHANOLKAR, S. R. and BUCHANAN, T. M. Humans respond predominantly with IgM immunoglobulin to the species-specific glycolipid of Mycobacterium leprae. J. Infect. Dis. 149( 1984)870-873.
36. YOUNG, R. A. and ELLIOTT, T. J. Stress proteins, infection and immune surveillance. Cell 59( 1989)5-8.
1. M.Sc; M.-C. Department of Parasitology, Microbiology and Immunology, Faculty of Medicine of Ribeirão Preto-University of São Paulo, 14049-900 Ribeirão Preto, SP, Brazil.
2. Ph.D., Department of Parasitology, Microbiology and Immunology, Faculty of Medicine of Ribeirão Preto-University of São Paulo, 14049-900 Ribeirão Preto, SP, Brazil.
Reprint requests to Dra. M.-C. Roque-Barreira.
Received for publication on 26 September 1994.
Accepted for publication in revised form on 2 February 1995.