- Volume 63 , Number 2
- Page: 249–58
Biometals in skin and sera of leprosy patients and their correlation to trace element contents of M. leprae and histological types of the disease; a comparative study with cutaneous tuberculosis
ABSTRACT
The present study has provided information on the biometal contents of killed and dried Mycobacterium leprae as well as dermal granulomas induced by the invading mycobacteria in various histological types of leprosy patients. For comparison, the biometal contents of the contralateral leprosy-unaffected skin of the same patients also were measured. The study also reports changes of scrum levels of the biometals in these patients which were compared with those in healthy control subjects and patients with skin tuberculosis. These data show that M. leprae is rich in zinc. During the course of the evolution of the disease there is gross alteration of the dynamics of the inflammatory cell population that infiltrates into leprosy granulomas, resulting in the alterations of trace element contents of the disease-affected skin lesions. Interestingly, the changes of the biometal contents in the granulomas of the patients with skin tuberculosis are similar to those in leprosy patients. It is postulated that the significant decrease of the contents of copper, zinc, iron, calcium and magnesium in the disease-affected skin in comparison to that of the contralateral healthy skin is a local effect, perhaps due to erosion or influx of biometal-deficient inflammatory cells into the affected skin with eventual loss of connective tissue of skin and mobilization of tissue-bound microelements into the vascular compartment. On the contrary, the changes in biometal levels in the sera of leprosy patients appear to be a general effect perhaps due to the release of interleukin-1, a product of inflammatory cells, causing hypercupremic, hypozincemic and hypoferremic responses in the hosts. Moreover, growth and multiplication of M. leprae, especially in polar lepromatous leprosy patients with a high bacillary load, demand essential biometals which may be mobilized into the bacterial bodies f rom the hosts. This perhaps results in the change in the homeostasis of the essential biometals in the hosts.RÉSUMÉ
La présente étude a fourni des informations quant au contenu biométallique de Mycobacterium leprae tués et séchés ainsi que de granulomes dermiques induits par des mycobactérics chez des patients présentant différents types histologiqucs de lèpre. On a également mesuré pour comparaison le contenu biométallique de la peau non atteinte par la lèpre chez les mêmes patients. L'étude rapporte également des modifications de taux sériques de biométaux chez ces patients, par comparaison avec ceux mesurés chez des témoins en bonne santé et des patients présentant une tuberculose cutanée. Ces données montrent que M. leprae est riche en zinc. Il y a, durant l'évolution de la maladie, une modification importante de la dynamique de la population cellulaire inflammatoire, qui infiltre les granulomes lépreux, résultant en des altérations du contenu en éléments des régions de la peau affectées par la maladie. Il est intéressant de noter que les modifications du contenu en biométaux au niveau des granulomes chez les patients présentant une tuberculose cutanée sont similaires à ceux observés chez les malades de la lèpre. On émet l'hypothèse que la diminution significative du contenu en cuivre, zinc, fer, calcium et magnésium de la peau atteinte par la maladie, en comparaison avec la peau saine, est un effet local, peut-être dû à l'érosion ou à l'arrivée de cellules inflammatoires déficientes en biométaux au niveau de la pacu atteinte, avec perte de tissu conjonctif et mobilisation de micro-éléments dans le compartiment vasculairc. Au contraire, les modifications des taux de biométaux dans le scrum de malades de la lèpre semblent être un effet général, peut-être dû à la libération d'interleukine-1, produite par les cellules inflammatoires, causant une augmentation du cuivre et une diminution du zinc et du fer sériques chez les patients. De plus, la croissance et la multiplication de M. leprae, particulièrement chez les patients présentant une lèpre lépromateusc polaire avec une charge bacillaire élevée, demandent des biométaux essentiels qui puissent être transférés de l'hôte aux bactéries. Ceci résulte peut-être dans une modification de l'homéostasie des métaux essentiels chez l'hôte.RESUMEN
El présenle estudio proporciona información sobre el contenido de biometales encontrado en Mycobacterium leprae muerto y desecado, y en los granulomas dérmicos inducidos por la micobacteria en varios tipos histológicos de la lepra. Para comparación, se midió el contenido en biometales de una región contralateral de la piel no afectada por la lepra en los mismos pacientes. El estudio también reporta los cambios en los niveles de biometales en el suero de estos pacientes en comparación con los encontrados en individuos sanos y en pacientes con tuberculosis de la piel. Estos datos muestran que M. leprae es rico en zinc. Durante el curso de la enfermedad hay una marcada alteración en la dinámica de la población de las células inllamatorias que infiltran los granulomas de la lepra, dando como resultado alteraciones en el contenido de los elementos traza de las lesiones de la piel afectadas por la enfermedad. Interesantemente, los cambios observados en el contenido de biometales en los granulomas de los pacientes con tuberculosis de la piel fueron similares a los encontrados en los pacientes con lepra. Se propone que la significativa disminución en el contenido de cobre, zinc, hierro, calcio y magnesio en la piel afectada por la enfermedad (en comparación con el contenido de estos biometales en las regiones sanas de la piel contralateral) es un efecto local debido a la erosión causada por el influjo de células inflamatorias deficientes en biometales que conduce a la pérdida de tejido conectivo de la piel y a la movilización de los microelementos unidos a tejido hacia el compartimento vascular. Por el contrario, los cambios en los niveles de biometales en el suero de los pacientes con lepra reflejan un efecto sistémico debido quizá a la liberación de interleucina 1, un produeto de las células inflamatórias que puede ocasionar respuestas hipercuprémicas, hipozinquémicas e hipoferrémias. Adernas, cl crecimiento y la multiplicación de M. leprae, especialmente en los pacientes lepromatosos con una alta carga bacteriana, requière de biometales csencialcs los cuales podrían ser movilizados a los microorganismos a partir dei huésped, ocasionando câmbios en la homeostasis de los biometales csencialcs en este último.Like all other organisms, bacteria require nutrients for their growth, enzyme activities, redox system, transport mechanism and synthesis of cell wall (5). In chronic, bacterial granulomatous diseases, such as leprosy and tuberculosis, human hosts provide all nutrients including minerals which are essential for the growth of the pathogenic mycobacteria. This may affect the homeostasis of the trace elements in the hosts. Earlier Saha and Rao reported elevated blood copper and lowered blood zinc and iron concentrations in lepromatous leprosy patients(13)
Leprosy is characterized by a spectrum of clinical manifestations, resulting from the interactions between the host immune response and the invading Mycobacterium leprae. Although the skin is the target tissue of intense mycobacterial and chronic inflammatory activities, there is little information on the concentrations of trace elements in the dermal granuloma of the patients, especially over the spectrum of the disease-- Also, no information is available on the concentrations of trace elements in M. leprae and in the inflammatory cells that infiltrate into the granuloma. In the present study an attempt has been made to a) estimate the contents of copper, zinc, iron, calcium, and magnesium in the dermal granuloma and healthy contralateral skin of leprosy patients over the spectrum of the disease; b) estimate the serum concentrations of these elements in these patients as well as in healthy controls; c) estimate the contents of these elements in M. leprae; d) find out any change in the homeostasis of these elements in the sera and target skin tissues of the patients; and e) find any correlation between the altered homeostasis of trace elements and the disease spectrum.
MATERIALS AND METHODS
Human materials
A total of 55 untreated leprosy patients (42 males, 13 females; mean age 33.4 ±13.1 years) were included in the study from the Urban Leprosy Centre, Safdarjung Hospital, New Delhi, India, during the period between June 1990 and March 1991. The mean duration of illness was 33.6 ± 12.1 months. The diagnosis of leprosy was established by clinical findings, slit-skin smears and lepromin testing. Histological classification (Ridley and Jopling) was made (12).
For comparison 20 normal healthy volunteers (15 males, 5 females; mean age 35.7 ± 15.4 years) were included in the study. All patients and controls belonged to the low socioeconomic group with a per capita monthly income below Rupees 150/- (11).
Another type of granulomatous disease was taken as another type of control: 7 untreated patients with skin tuberculosis (4 males, 3 females; mean age 36.35 ± 25 years). Five of them had lupus vulgaris (cheek, elbow, heel and neck) and 2 had scrofuloderma (neck and inguinal region).
The diagnosis was based on clinical, histological and bacteriological examinations.
Sample collection
Tissue samples. Two punch biopsies (5-mm diameter) were taken under local anesthesia from each patient (leprosy and tuberculosis), one from the margin of the skinlesion and another from the leprosy-unaffected skin of the contralateral side.
Serum samples. Ten ml of fasting bloodwas collected from all patients and controlsin clean, metal-free, plastic specimen vials(Tarsons, India). The sera were separatedand stored in clean plastic vials at -70°Cuntil use.
M. leprae . For estimation of the contentsof the elements in the bacterial bodies, aknown volume of armadillo-derived lep-romin (World Health Organization) containing 1.6 x 108 Al. leprae per ml was cen-trifuged. The bacteria were washed thoroughly with distilled water, then dried andstored until use.
Chemical reagents and laboratory wares
Metal-free water (equivalent conductancegreater than 18 Mhos), high purity chemcals (Specpure, Suprapure, analytical orelectron microscopy grade) and laboratorywares made of PFA teflon, polypropyleneor high-density polyethylene were used.Laboratory wares were cleaned by soakingin 20% nitric acid (analytical grade) for 7days, and rinsed repeatedly thereafter withdistilled water (eight changes) before use.
Tissues and M. leprae processing
Ninety-four skin biopsies were collectedfrom 55 leprosy patients; 55 samples weretaken from lesions, the remaining 39 samples were collected from the apparentlyhealthy contralateral skin of the same patients. Sixteen contralateral healthy skinsamples could not be collected because ofdisseminated disease in 16 patients [12 lepromatous leprosy and 4 erythema nodosumleprosum (EN L) patients]. Seven samples ofdermal granuloma from 7 patients with skintuberculosis and seven skin samples fromtheir contralateral healthy skin areas weretaken.
The skin samples were dehydrated in100% ethanol and dried at 100°C for 2 hr.The dry weights were recorded, and thesamples were then transferred into speciallyfabricated Teflon PTFE digestion bombs of 3-ml capacity (The Figure). Suprapure nitricacid (500 µl; E. Merck, Germany; boilingpoint 120°C) was added. The bombs weretightly sealed and digestion of the tissueswas carried out in a hot-air oven at 90°Cfor 2 hr. The bombs were then cooled atroom temperature for 4 hr and the analyteswere transferred to Eppendorff tubes ofknown weights. From the final weights ofthe Eppendorff tubes, the quantities of thedigested materials were determined. Forquality control, sample blank and analytes(certified reference material of fish flesh homogenate; MA-A-2; International AtomicEnergy Agency, Vienna, Austria), preparedunder similar conditions, were employed.All digested materials were stored at 4°C forfurther analysis. A known number of M.leprae were digested in the bomb as described above.
The figure. Diagram of a 3-mI PTFE Teflon didisseminated disease in 16 patients [12 lepgestion bomb fabricated at the Defense Metallurgical romatous leprosy and 4 erythema nodosum Research Laboratory, Hyderabad, India. The conical leprosum (ENL) patients]. Seven samples of vessel had a 5-mm thick wall with an outer diameter dermal granuloma from 7 patients with skin of 10 mm and a depth of 55 mm; screw thread reached
Element analysis
The digested samples were diluted in triple-distilled water in suitable proportions.Copper, zinc, iron, calcium and magnesium in the digested tissue and M. leprae in theserum samples were estimated by a direct-current, plasma, atomic emission spectrom-eter using an argon plasma [AFL/BeckmanSpectrospan V Direct Current Atomic Emission Spectrometer (DCP-AES)]. The results were compared with high puritystandards containing all elements (1000 pg/ml) (Sigma Chemical Co., St. Louis, Mis-souri, U.S.A.) after diluting in suitable proportions.
Statistical evaluation
The results were grouped according to thehistological classification of leprosy and themean, standard deviation and range werecalculated. The Student's t test was used todetermine statistical significances.
The data also were evaluated by multi-variate discriminate analysis to assess theprobability of correctly determining the histological types of the patients by the levelsof the elements in the sera and dermal granulomas. All statistical analyses were per-formed using the STATGRAPHICS statis-tical package.
RESULTS
Human materials
Leprosy patients. Histological classifi-cation of the patients showed that there were9 tuberculoid (TT), 13 borderline tubercu-loid (BT), 10 midborderline (BB), 7 bor-derline lepromatous (BL), and 16 polar lep-romatous (LL) patients, including 4 patientswith ENL. The bacterial index (BI) rangedfrom 0 to 3+ in BB, 1+ to 4+ in BL, and2+ to 5+ in LL patients, including the ENLcases; 9 TT and 13 BT cases were bacteri-ologically negative.
Element contents of granulomatous andnormal skin of leprosy patients
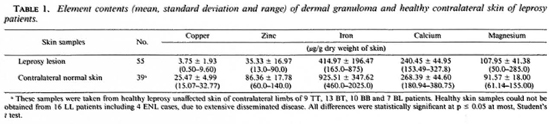
Table 1 shows a significant decrease inthe mean copper, zinc, iron and calcium anda significant increase of the mean magne-sium contents of leprosy-affected dermalgranuloma in comparison to that of the con-tralateral healthy skin samples collectedfrom the 55 patients.
Table 2 shows the decreasing trends ofcopper, iron and calcium in skin lesions of leprosy patients from the polar tuberculoid to the lepromatous end of the disease spectrum. Increasing trends were observed in the zinc and magnesium contents in the lesions of the leprosy patients from the TT end to LL end. Another striking finding was the increased contents of copper, zinc, iron and calcium in lesions of the patients during ENL, in comparison to those in the LL patients without any reaction.
Element analysis in serum samples
Table 3 shows a significant increase of the mean serum copper level in the leprosy patients in comparison to the controls. On the other hand, the mean serum levels of zinc, iron, calcium and magnesium were significantly less than those in the controls.
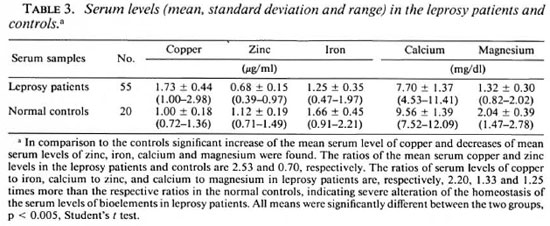
Table 4 shows the variations of the scrum levels of the above elements in the various histological forms of leprosy. There was an increasing trend of mean serum concentrations of copper and magnesium in leprosy patients from the tuberculoid to the lepromatous end of the disease spectrum. Decreasing trends were observed in the mean serum levels of zinc, iron and calcium in the leprosy patients from the TT to LL end. Only the mean serum copper and iron levels increased in the ENL patients in comparison to LL patients without any reaction.
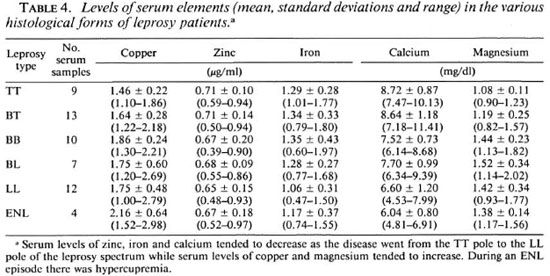
Table 5 shows the contents of trace elements in M. leprae: 1.6 x 108 bacilli (weighing 6.4 µg) had 20, 1400, 50 and 50 ng of copper, zinc, iron and magnesium, respectively.

Element contents of granulomatous and normal healthy skin of patients with skin tuberculosis (controls)
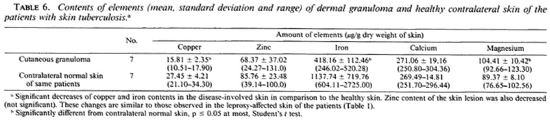
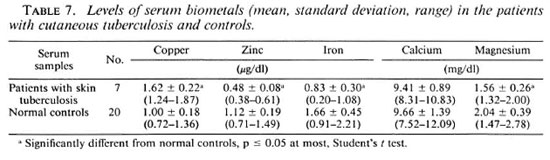
Table 6 shows that the differences between the biometal contents in the dermal granuloma and healthy contralateral skin of the patients with skin tuberculosis were similar to those observed in the leprosy patients (Table 1). However, the decrease in the copper, zinc, iron and calcium contents of leprous granuloma in comparison to the contralateral healthy skin was more than the decrease found in the tuberculosis granuloma.
DISCUSSION
Alterations of serum element levels in leprosy patients
This study has revealed a gross alteration of the homeostasis of micro-elements in the skin lesions and blood of leprosy patients (Tables 1 and 3). Table 5 shows an accumulation of trace elements, especially zinc, in the bacterial (M. leprae) bodies. In LL leprosy the bacterial load may be as high as 1011 (3). The weight of 1011 M. leprae is about 4 mg (weight of a bacterium is 3.9 ± 1.0 x 10-14 g) (3). Thus, the amounts of copper, zinc and iron accumulated in 1011 M. leprae are estimated to be 1,248, 880 and 31 µg, respectively (Table 5), which certainly have been provided by the hosts. This could create alterations in the normal contents of the various trace elements in the blood and skin because these biometals are essential tissue constituents. Table 4 shows the maximum reductions of the mean serum levels of zinc, iron and calcium in the LL patients with a high bacillary load of 2 + to 5 + . This provides support to the view that host tissues provide micro-nutrients to the invading M. leprae for their growth.
Alterations of contents of elements in leprosy-affected skin
The dermis of the leprosy-affected patient skin is made up of collagen and elastin. Copper plays a critical biochemical role in the maturation, as well as maintenance, of the structural integrity ofthese two proteins (5,9). Within dermal granulomas of LL patients there is a breakdown ofdermal extracellular connective tissue with invasion of M. leprae which are rich in trace elements (Table 5). There is also infiltration of large numbers of various copper-deficient, chronic inflammatory cells, such as lymphocytes and macrophages.
We have studied the concentrations of various trace elements of these cells within leprosy granuloma by an electron microscope coupled with a computerized X-ray spectroscope. (The results of these studies are out of the scope of the present study and will be published elsewhere.) From the results of these studies it has been postulated that the observed reduction of the copper contents in the leprosy-affected dermal granulo.na (Table 1) perhaps indicates: a) a reduction of the level of copper containing lysl-amino-oxidase enzyme in the affected skin, causing inhibition of crosslinking of newly synthesized collagen fibrils (5,14) and b) an influx of trace-element-dcficicnt inflammatory cells into the leprosy granuloma (unpublished data). Both copper and zinc are constituents of superoxide dismutase (SOD) enzyme of the host (5) which plays an important role in the killing of intracellular M. leprae (4). M. leprae also possesses SOD (19). The observed high contents of copper and zinc in leprosy bacilli (Table 5) provide further support to our postulate that host tissues (leprosy granuloma) provide biometals to the acid-fast bacilli. Low copper and zinc contents in the dermal granuloma of the leprosy patients (indicating low SOD activity of the granuloma) vis-a-vis high copper and zinc contents of M. leprae (indicating high SOD activity of the intracellular M. leprae) may be contributing factors toward the uncontrolled intracellular multiplication of the bacilli in LL patients. The observed elevation of the mean serum copper level in our leprosy patients (Table 3) may be due to the degeneration of collagen tissue of the disease-afTected skin and eventual mobilization of the dermal collagen-copper into the vascular compartment
Zinc is involved in the formation and activation of more than 200 enzymes, including superoxide dismutase and nucleic acid polymerases (5). Metal ions are present at the active sites of many enzymes, even proteins associated with nucleic acids and a transcription factor for 5 S RNA gene containing nine zinc binding domains. Zinc also has an important role in the cell-mediated immune response (4). Thus, its deficiency in the serum and dermal granuloma of leprosy patients may lead to the breakdown of cellmediated immunity, especially T-helper cell functions (8). The observed decrease in the serum levels of zinc in our leprosy patients (Table 3) as well as the decreased zinc contents in the leprosy-affected skin may be due to the accumulation of a large amount of zinc inside the bacterial bodies of M. leprae (Table 5). However, this view may be challenged because of the presence of a large number of biometal-containing M. leprae in the dermal granuloma, especially in samples from the LL patients. Table 2 supports this view, because the zinc contents in the lesions of BL and LL patients with high bacterial density were more than those in the TT and BT patients with low bacterial density.
An alternative explanation for the observed changes in the serum levels of copper, zinc and iron in our leprosy patients (Table 3) is that production of interleukin-1 in a chronic inflammatory disease such as leprosy causes increased blood copper, and decreased blood zinc and iron (18). Thus, low zinc in infectious diseases is not due to body loss (l6), and serum iron is depressed in all infectious diseases (1).
The present study has shown low serum iron levels in all histological forms of leprosy in comparison to the controls with a remarkable decline in the LL form of the disease (Tables 3 and 4). On the other hand, the iron contents in the leprosy-affected skin samples taken from LL and BL patients, although less than that in TT patients, was more than that in BB patients (Table 2). This increased iron content of LL granulomas is accounted for by the presence of a large number of iron-containing M. leprae in the skin lesions of BL and LL patients.
It is well known that any acute inflammatory state can result in a shift of serum zinc from the intravascular to extravascular compartment. This also may account for the observed decrease in the serum zinc level in our ENL patients. Also, according to Kochen, low serum and tissue iron concentrations are an adaptive host response for making these essential nutrients unavailable to the invading microorganism (6). Copper, zinc, calcium and iron are important constituents of red blood cells; hence vascularity of a lesion can influence the concentrations of these elements in the various tissues. In the present study high biometal contents in the dermal lesions of our ENL patients (Table 4) may be a direct reflection of high concentrations of red blood cells in these areas. Also, according to Vohora low serum iron is a characteristic feature of leprosy and is more pronounced in the reactive phase of leprosy (20). Vohora reported that with the rising bacterial load as the disease spectrum shifts from TT to LL, there is a progressive reduction of serum iron level 20). Table 4 also shows a progressive decline of the mean serum iron levels as the disease moves from the BT to the LL end of the spectrum.
Tuberculosis of skin
The changes of the biometal concentrations in the cutaneous granuloma of the patients with skin tuberculosis were similar to those found in the leprosy patients. However, the decreases of copper, zinc, iron and calcium in the leprosy granulomas in comparison to the healthy contralateral skin were more than in the patients with skin tuberculosis (Tables 1 and 6). Table 7 shows that the alterations of the biometals in the sera of the patients with skin tuberculosis were similar to those found in the sera of leprosy patients (Tables 3 and 7).
Multivariate discriminate analysis
This analysis was carried out to ascertain if the data on the elements in the sera and skin lesions are able to predict the histomorphology of the disease correctly. Table 8 summarizes the results ofthe discriminate analysis ofeorrectly predicted values for different elements, cither singly or in combination for different samples. The analysis indicates that lesional copper or a combination of lesional copper, zinc, iron, calcium and magnesium, either alone or in combination with their scrum levels and/or contralateral healthy control dermal element contents, can be helpful in correctly predicting (100%) the differences between controls and leprosy patients; while zinc, iron, calcium and magnesium levels can account for 91% of the correct prediction.
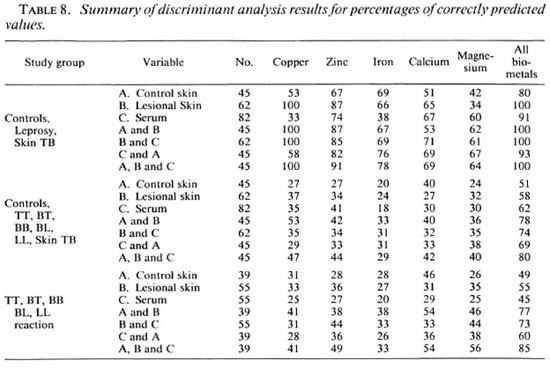
However, none of the parameters alone can differentiate successfully between the different histological types of leprosy. The percentage of predictability for copper, zinc, iron, calcium and magnesium levels together was only 45%; the levels of these btometals in the lesions could also account for only 55% of the correct prediction. Further, all elements in the sera and lesions taken together can provide a 73% correct prediction. Nevertheless, contribution of serum and lesional elements as well as that of contralateral skin controls for all five elements (total 15 parameters), considered together, can provide a correct predictability ofup to only 85%.
REFERENCES
1. BEISEL, W. R. Single nutrients and immunity. Am. J. Clin. Nutr. 35 Suppl.(1982)417-468.
2. 258 International Journal of Leprosy 1995 2. BRITTON, W. J. Immunology of Leprosy. Trans. R. Soc. Trop. Med. Hyg. 87(1993)508-514.
3. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy. Edinburgh: Churchill Livingstone, 1979, p. 49.
4. CORMAN, L. C. The relationship between nutrition, infection and immunity. Med. Clin. North Am. 69(1985)19-531.(72 ref.)
5. FRAUSTO DA SILVA, J. J. R. and WILLIAMS, R. J. P. The Biological Chemistry of Elements; the Inorganic Chemistry of Life. Oxford: Clarendon Press, 1993, p. 312.
6. KOCHEN, I. Nutritional regulation of antibacterial resistance. In: Microbiology. Schlessinger, D., ed. Washington, D.C.: American Society of Microbiology, 1974, pp. 273-280.
7. LEOPOLD, I. H. Zinc deficiency and visual impairment. Am. J. Ophthalmol. 85(1978)871-875.
8. MYRVIK, Q. N. Nutrition and immunology. In: Modern Nutrition in Health and Diseases. 7th cdn. Stils, M. E. and Young, V. R., eds. Philadelphia: Lea and Febiger, pp. 585-616.
9. O'DELL, B. L. Role of iron and copper in connective tissue biosynthesis. Philos. Trans. R. Soc. London [Biol.] 294(1981)91-104.
10. OON, B. B., KHONG, K. Y., GREAVES, M. W. and PLUMMER, V. M. Trophic skin ulceration of leprosy: skin and scrum zinc concentration. Br. Med. J. 2(1974)531-533.
11. RAO, K. N. and SAHA, K. Undernutrition in lepromatous leprosy. Part III. Altered levels of serum elements; their association with the disease and not with food deprivation. Lepr. Rev. 57(1966)311-316.
12. RIDLEY, D. S. and JOPLING, W. H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
13. SAHA, K. and RAO, K. N. Undernutrition in lepromatous leprosy. Part V. Several nutritional deficits in lepromatous leprosy patients co-infected with pulmonary tuberculosis. Eur. J. Clin. Nutr. 45(1989)117-128.
14. SEYER, J. M. and KANG, A. H. Structural proteins: collagen, clastin and fibronectin. In: Textbook of Rheumatology. 2nd edn. Kelley, W. N., Barris, E. D., Ruddy, S. and Sledge, C. B., eds. Philadelphia:
W. B. Saunders Company, 1985, pp. 211-235.
15. SHANKAR, A., GUPTA., S. B. and SHARMA, J. N. A study of serum and skin zinc in leprosy. Indian J. Dermatol. Venercol. Leprol. 42(1976)258-260.
16. SHER, R., SHULMAN, G., BAILY, P. and POLITZER, W. M. Serum trace elements and vitamin A in leprosy subtype. Am. J. Clin. Nutr. 34(1981) 1918-1924.
17. SHESKIN, J. and ZEIMER, R. In-vivo study of trace elements in leprous skin. Int. J. Dermatol. 15(1981)145-150.
18. WATSON, S., BULLOCK, W., NELSON, K., SCHAUF, V., GELBER, R. and JACOBSON, R. Interleukin-1 production by peripheral blood mononuclear cells from leprosy patients. Infect. Immun. 45(1984)787-789.
19. WHEELER, P. R. and GREGORY, D. Superoxide dismutase, peroxide activity and catalase in Mycobacterium leprae. J. Gen. Microbiol. 121(1980)457-464.
20. VOHORA, S. B. Elementology of leprosy. Indian J. Lepr. 58(1986)451-460.
1. Ph.D.; Department of Electron Microscopy, Institute of Pathology, Indian Council of Medical Research, New Delhi, India.
2. M.D., Department of Electron Microscopy, Institute of Pathology, Indian Council of Medical Research, New Delhi, India.
3. M.D., Department of Microbiology, National Institute of Communicable Diseases, Delhi 110054, India.
4. M.Sc, M.B.B.S., Ph.D. (Penn., U.S.A.), Department of Immunology, Vallabh Bhai Patel Chest Institute, Delhi University, Delhi 110007, India.
Reprint requests to Dr. Saha, 45A Sova Bazar Street, Calcutta 700005, India.
Received for publication on 27 July 1994;
Accepted in revised form on 14 February 1995.