- Volume 63 , Number 2
- Page: 259–64
Activity of combinations of dapsone, rifampin, minocycline, clarithromycin, and sparfloxacin against M. leprae-infected mice
ABSTRACT
In these studies we evaluated the activity of low levels of five antimicrobials against Mycobacterium leprae'-infected mice when administered singly and in all possible twoand three-drug combinations. Antibiotics studied were: dapsone (D) 0.0001% in the diet, rifampin (R) 20 mg/kg by gavage once monthly, minocycline (M) 0.004% in the diet, clarithromycin (C) 0.001% in the diet, and sparfloxacin (S) 5 mg/kg by gavage five times weekly. Singly each agent was found bacteriostatic (D + R) or partially bactericidal (M, C, and S) but not fully bactericidal. All 10 two-drug regimens were found at least bacteriostatic, 2 being "partially bactericidal" and 4 being "fully bactericidal." Of the 10 three-drug regimens, 9 were found "fully bactericidal" and the other "partially bactericidal." We conclude that combinations of antibiotics active against M. leprae are generally additive in combination.RÉSUMÉ
Nous avons evalué dans ces études l'activité, vis-àvis de souris infectées par Mycobacterium leprae, de faibles taux de cinq antimicrobiens administrés isolément ou dans toutes les combinaisons possibles de deux ou trois produits. Les antibiotiques étudiés étaient: la dapsonc (D) 0.0001% dans la nourriture; la rifampicine (R) 20 mg/kg par gavage une fois par mois; la minocycline (M) 0.004% dans la nourriture; la clarithromycine (C) 0.001% dans la nourriture; et la sparfloxacine (S) 5 mg/kg par gavage cinq fois par semaine. Chaque agent isolément a été trouvé sit bactériostatique(D + R)ou partiellement, mais non complètement bactéricide (M, C et S). Les dix combinaisons de deux produits ont été au moins trouvées bactériostatiques, deux étant partiellement bactéricides et quatre l'étant complètement. Parmi les 10 combinaisons à trois produits, 9 ont été trouvées complètement bactéricides et le dernier partiellement bactéricide. Nous en concluons que les antibiotiques actifs contre M. leprae ont généralement une puissance qui s'additionne quand ils sont utilisés de manière combinée.RESUMEN
En este estudio se evaluó la actividad de cinco antimicrobianos administrados en niveles bajos en ratones infectados con Mycobacterium leprae. Los fármacos se administraron aislados o en combinaciones de dos o tres drogas. Los antibióticos fueron: dapsona (D) al 0.0001% en la dieta, rifampina (R) a 20 mg/kg por via oral una vez al mes, minociclina (M) al 0.004% en la dieta, claritromicina (C) al 0.001% en la dieta, y esparlloxacina (S) a 5 mg/kg por via oral cinco veces a la semana. Administrados en forma aislada, cada agente resultó bacteriostático (D+R) o parcialmente bactericida (M, C, y S), pero no totalmente bactericida. Las diez combinaciones de 2 drogas usadas fueron al menos bacteriostáticas, siendo 2 parcialmente bactericidas y 4 totalmente bactericidas. De las 10 combinaciones de 3 drogas, 9 fueron completamente bactericidas y una parcialmente bactericida. Concluimos que los efectos de las combinaciones de antibióticos activos contra M. leprae son generalmente aditivos.There are two reasons to use combinations of antibiotics to treat leprosy. The first is in order to prevent drug resistance. The second is the opportunity to improve on the killing of Mycobacterium leprae obtained by single agents.
Although to date we have been unable to measure the prevalence of naturally occurring antimicrobial resistance of M. leprae among a sensitive bacterial population to any single agent, experience with antibiotics and other bacteria has demonstrated this to be in the range of 1 in 105 - 109. Since untreated lepromatous leprosy patients harbor as many as 10" viable M. leprae (23), the need to combine antibiotics in the therapy of lepromatous disease is self-evident. Furthermore, clinical experience with the monotherapy of tuberculosis with several agents (12) and leprosy with dapsone (l3,20) has resulted in unacccptably high rates of treatment failure owing to the selection of drugresistant strains.
Because multidrug therapy has now been established as the therapy of lepromatous leprosy, a by-product ofthat therapy could, as was accomplished for tuberculosis therapy, be enhanced mycobacterial killing and, hence, the ability to effectively reduce the duration required for treatment. This might especially be possible with the emergence in the past few years of antibiotics from three classes [tetracyclines (minocycline), macrolides (clarithromycin), and fluoroquinolones (ofloxacin/sparfloxacin)] which, unlike only rifampin and not dapsone and clofazimine, are convincingly bactericidal for M. leprae in man (8, 11, 14, 21, 25, 27) In any event, the need to assure that combination antimicrobials are at least not antagonistic in their effect on the killing of M. leprae is important.
Unfortunately, there is limited experimental data on the efficacy of combinations, particularly of these newer agents, against M. leprae germane to this issue. However, there are some: Shcpard (22) previously demonstrated in extensive studies in mice that combinations of two antimicrobials, first dapsone, clofazimine, and ethionamide, were generally additive and not antagonistic in their antimicrobial effects. Later, when rifampin was also combined with these agents, it was found that in 16 of 22 instances a greater antimicrobial effect was produced than that by the most effective single agent (24), again suggesting that combinations of antimicrobials are generally additive in their activity for M. leprae. Although there are several reports (6, 15' 16' 28' 29) of the activity of combinations of these newer agents against M. leprae in both normal and nude mice, finding them generally additive in combination, these studies report on only a few combinations. Of especial interest, in the heavily M. leprae -infected neontally thymectomized Lewis rat we (7) recently found that while several agents administered singly and in combination did not eliminate viable M. leprae, a combination of rifampin plus minocycline, as well as the combinations of rifampin plus ethionamide and rifampin plus ofloxacin did.
Because there are no comprehensive studies to date on the activity of combinations of antimicrobials against M. leprae, which include active agents from the three newer classes of bactericidal antibiotics, the current study was initiated. Unfortunately, studies of antimicrobial combinations can require very large numbers of mice and, hence, we limited the number of older antimicrobials studied to two, dapsone and rifampin. Dapsone was selected for study because it has been used longest and remains the major agent used to treat leprosy worldwide. Rifampin was selected because, of the older agents, it is the only one that is convincingly bactericidal in patients (patients(21, 26, 27). The choice fluoroquinolone, sparfloxacin, was largely arbitrary, but in some studies it has been found to be more active against M, leprae in mice than equivalent amounts of other fluoroquinolones. (4,9,18)
METHODS
In these studies we attempted to utilize antimicrobials in mice in doses which alone were partially effective against M. leprae but not so potent as to obscure any observable additive effects in combination. For these purposes we chose dapsone (D) 0.0001% in the diet; rifampin (R) 20 mg/kg by gavage once monthly (days 60, 90, and 120 after foot pad infection); minocycline (M) 0.004% in the diet; clarithromycin (C) 0.001% in the diet and sparfloxacin (S) 5 mg/kg by gavage five times weekly.
In these studies groups of female BALB/c mice (Jackson Laboratories, Bar Harbor, Maine, U.S.A.) were infected in both hind feet with 5000 M. leprae and treated from day 60-150 thereafter either as controls (no drugs) or with these five agents singly and in all possible two- and three-drug combinations (10 each). At the completion of therapy and 3 and 7 months subsequently, the number of M. leprae in two mice (four feet) was enumerated microscopically for all treatment groups. Definite multiplication was considered to have occurred if the number of M. leprae/foot pad found therein was > 105.
By these criteria, regimens were considered inactive if M. leprae had multiplied by the completion of therapy and bacteriostatic if M. leprae had not multiplied by then but had multiplied 3 and 7 months subsequently. A delay in M. leprae multiplication which cannot be accounted for by drug accumulation may represent cither bactericidal or bacteriopausal activity, delays of several months suggesting that actual bactericidaltype activity had been observed (7). In these present studies, if M. leprae were found not to have multiplied at the completion of therapy or 3 months thereafter but had multiplied 7 months subsequently, activity was termed "partially bactericidal." Because none of the antimicrobial agents utilized in these studies appreciably accumulates in tissues and because the 3-month interval for the second foot pad harvest herein is the same period as the treatment interval, findings such as these imply that some bactericidal activity was observed. If M. leprae were found not to have multiplied at all three harvest intervals, activity was designated "fully bactericidal."
RESULTS
In the untreated control mice the initial 5000 M. leprae/foot pad inoculum multiplied to a level of 2.37 x lO5 foot pad by the completion of therapy and remained at approximately that level at the two subsequent harvests (The Tabic). All five antimicrobials administered singly were found active, M. leprae/foot pad < 105 at the completion of therapy (The Table). Three months subsequently in mice treated with two of the agents administered singly (dapsone and rifampin) but not clarithromycin, sparfloxacin, or minocycline, M. leprae had multiplied (The Table). By 7 months after the completion of therapy, M. leprae had convincingly multiplied in mice treated with each agent singly (The Table). Thus, we conclude that at the doses utilized in this study dapsone and rifampin were purely bacteriostatic, while minocycline, clarithromycin, and sparfloxacin were "partially bactericidal."
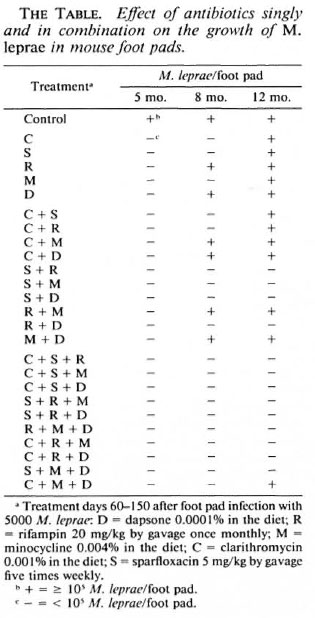
None of the 10 two-drug combinations resulted in M. leprae multiplication at the completion of therapy (The Table). At 3 months and 7 months subsequently, M. leprae had multiplied in four of the two-drug regimens (C + M, C + D, R + M and M + D; The Table), these regimens being found purely bacteriostatic. Four two-drug regimens (S + R, S + M, S + D and R + D) resulted in no observed M. leprae multiplication at either post-treatment harvest interval (The Table), being found "fully bactericidal." Lastly, in 2 two-drug regimens (C + S and C + R) while M. leprae had not multiplied in mice 3 months after completion of therapy it had by 7 months (The Table), these being found to be "partially bactericidal."
In none ofthe 10 three-drug regimens had M. leprae multiplied cither by the completion of therapy or 3 months thereafter (The Table). In only one of these regimens (C + M + D) had M. leprae multiplied even 7 months after the completion of therapy (The Table). Thus, only this three-drug combination was found "partially bactericidal," while all of the nine other thrcc-drug combinations were determined to be "fully bactericidal."
DISCUSSION
Fortunately, in these studies we found that all five antimicrobial agents administered singly were active but none "fully bactericidal," allowing the opportunity to observe additive behavior when combinations were administered. Previously, we had determined the appropriate dose of each of these agents, except sparfloxacin, for the M. leprae isolate utilized in this study. Also, we (9) had found that sparfloxacin in doses of 15-300 mg/kg by gavage five times weekly was fully bactericidal for this isolate; others (10) had found in nude mice that 10 mg/kg was bactericidal, while 2 mg/kg was purely bacteriostatic. The finding herein that 5 mg/ kg was partially bactericidal confirms sparfloxacin's minimal bactericidal daily dose to be in the range of 5 mg/kg. This is in contrast to ofloxacin, where the comparable bactericidal daily dose in mice has been found to be several-fold greater (4, 9 ,18 ).
Although rifampin was found in these studies to be purely bacteriostatic, rifampin at the dose administered is generally profoundly bactericidal for M. leprae in mice (2.5.16.21.29) and in daiiy doses in leprosy patients (21, 26,27). The particular isolate utilized for these studies was chosen specifically for its relative insensitivity to the bactericidal action of rifampin. We (5) previously reviewed results that, unlike for dapsone where the minimal inhibitory concentration and the minimal bactericidal concentration are uniformly low, there is a considerable range for rifampin. In leprosy patients, although daily rifampin generally clears viable bacilli from the dermis in a few days (26,27), the fact that in the first two clinical trial patients given rifampin Rees found that viable M. leprae in one of these patients was present 17 days after the initiation of treatment, confirms that in fact such relatively rifampin-resistant strains clearly also exist in patients.
The finding that, unlike any of the agents administered singly, 4 of the 10 two-drug regimens and 9 of the 10 three-drug regimens were "fully bactericidal" demonstrates once again that the activity of combinations of antimicrobial agents against M. leprae are generally additive.
At both the 8-and 12-month sampling intervals there were 2 of the 5 single agents (dapsone and rifampin) and 4 of the 10 twodrug regimens (C + M, C + D, R + M, M + D) and none of the 10 three-drug regimens wherein multiplication had occurred, resulting in what we consider purely bacteriostatic activity. It is noteworthy that for all four of the two-drug regimens found to be purely bacteriostatic, "partially bactericidal" activity was obtained for one of the two administered agents when utilized singly, implying that, at the very low antimicrobial level selected in this study, there were a few instances of possible antimicrobial antagonism. It is further noteworthy that for the only three-drug combination (C + M + D) which was found "partially bactericidal" but not "fully bactericidal," all three of its component two-drug combinations were less active than one of the drugs administered singly, possibly antagonistic, and only bacteriostatic.
Because of the consistently profound bactericidal activity of rifampin for M. leprae both in mice (2, 5, 16, 21, 29) and, more importantly, in affected patients (21, 26 ,27), it is particularly important for any future clinical application that candidate combination antimicrobials be found to not antagonize its action. Of potential relevance to this issue is the finding that rifampin plus another macrolide antibiotic, erythromycin, were demonstrated previously in vitro to be, among several other antimicrobial combinations, the most bactericidal against Staphylococcus aureus (l9), and that rifampin plus tetracycline increased the survival rate of mice injected intraperitoneally with gram-negative organisms (Salmonella typhimurium, Escherichia coli, Proteus vulgaris, and Shigella dysenteriae) compared to the survival rate when either drug was used alone (1).
Ideally, in studies to identify and distinguish additive, synergistic, and antagonistic effects of antibiotics in combination, the doses chosen would ideally achieve an overall ED50. Unfortunately, the mouse foot pad system does not allow for precise estimation of an ED50, and such dosing therein would certainly obscure the ability to observe an additional activity of combinations by the mouse foot pad methods utilized in these present studies. In these present studies, the only antimicrobial studied in combination with rifampin in which there was any possible evidence for antagonism was minocycline. This is in contrast to our previous studies wherein it was found, both in mice (6) and in neonatally thymectomized Lewis rats (7), that the combination of rifampin and minocycline, when administered at higher levels which approximate those actually obtained in patients, were found additive, suggesting that there may be differences in this combined antimicrobial effect when rifampin is combined with minocycline at high and low levels. In fact, for several other bacterial species rifampin in combination with another antimicrobial agent, trimethoprim, was found synergistic at high levels, while not so or even antagonistic at low levels (3,17). Thus, we have reason to believe that the possible antagonism demonstrated between rifampin and minocycline herein is a function of the very low doses of antimicrobials which were utilized in this study and would not be relevant to the levels actually achieved in treated leprosy patients.
In conclusion, we generally found evidence for additive activity of combinations of antimicrobials for M. leprae and little evidence for possible antagonism. Because combinations of the newer antimicrobials, minocycline, clarithromycin, and fluoroquinolones, are being considered in combination with rifampin for the treatment of leprosy, these results are encouraging that such combinations will not only prevent the emergence of resistant M. leprae but will also enhance bacterial killing and even potentially shorten the required duration of therapy for lepromatous leprosy patients.
Acknowledgment. This investigation was financially supported by a grant from the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A.
REFERENCES
1. ARIOLI, V., PALLANZA, R., NICHOLIS, F. B. and FURESZ, S. Experimental data on the interaction of Drug Combinations between rifampicin and tetracycline; progress in antimicrobial and anticancer chemotherapy. In: Proceedings of the 6th International Congress of Chemotherapy. Tokyo: University of Tokyo Press, 1977, p. 339.
2. COLSTON, M. J., HILSON, G. R. and BANERJEE, D. K. The "proportional bactericidal test": a method for assessing bactericidal activity in drugs against Mycobacterium leprae in mice. Lepr. Rev. 49(1978)7-15.
3. FARRELL, W., WILKS, M. and DRASAR, F. A. The action of trimethoprim and rifampicin in combination with gram-negative rods resistant to gentamicin. J. Antimicrob. Chemother. 3(1977)459-462.
4. FRANZBLAU, S. G., PARRILLA, M. L. and CHAN, G. P. Sparfloxacin is more bactericidal than ofloxacin against Mycobacterium leprae in mice. Int. J. Lepr. 61(1993)66-69.
5. GELBER, R. H. The killing of Mycobacterium leprae in mice by various dietary' concentrations of dapsonc and rifampicin. Lepr. Rev. 57(1986)347-353.
6. GELBER, R. H. Activity in minocycline in Mycobacterium leprae-infccXcd mice. J. Infect. Dis. 156(1987)236-239.
7. GELBER, R. H. The chemotherapy of lepromatous leprosy: recent developments and prospects for the future. Eur. J. Microbiol. 1994 (in press).
8. GELBER, R. H., FUKUDA, K., BYRD, S., MURRAY, L. P., Siu, P., TSANO, M. and REA, T. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 304(1992)91-92.
9. GELBER, R. H., IRANMANESH, A., MURRAY, L., SIU, P. and TSANG, M. Activities of various quinolone antibiotics against Mycobacterium leprae in infected mice. Antimicrob. Agents Chemother. 36(1992)2544-2547.
10. GIDOH, M. and TSUTSUMI, S. Activity of Sparfloxacin against Mycobacterium leprae inoculated into foot pads of nude mice. Lepr. Rev. 63(1992)108-116.
11. GROSSET, J.-H., JI, B., GUELPA-LAURAS, C.-C, PERANI, E. G. and N'DELI, L. N. Clinical trial of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
12. GROSSET, J.-H., JI, B. and ITO, T. The first joint THELEP-Sasakawa Memorial Health Foundation workshop on experimental chemotherapy of leprosy. Int. J. Lepr. 55(1987)807-813.
13. Ji, B. Drug resistance in leprosy; a review. Lepr. Rev. 56(1985)265-278.
14. Ji, B., JAMET, P.. PERANI, E. G., BOBIN, P. and GROSSET, J.-H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(1993)188-190.
15. Ji, N., PERANI, E. G. and GROSSET, J.-H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
16. Jt, 13., PERANI, E. G., PETINON, C. and GROSSET, J.-H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
17. KERRY, D. W., HAMILTON-MILLER, J. M. T. and BRUMFITT, W. Trimethoprim and rifampicin: in vitro activities separately and in combination. J. Antimicrob. Chemother. 1(1975)417-427.
18. MCDERMOTT-LANCASTER, R. D. and BANERJEE, D. K. Activity of Sparfloxacin against Mycobacterium leprae measured by the proportional bactericidal test. Int. J. Lepr. 61(1993)605-608.
19. PEARD, M. C, FLECK, D. G., GARROD, L. P. and WATERWORTH, P. M. Combined rifampicin and erythromycin for bacterial endocarditis. Br. Med. J. 4(1970)410-411.
20. PEARSON, J. M. H., REES, R. J. W. and WATERS, M. F. R. Sulfphone resistance in leprosy: a review of one hundred proven clinical cases. Lancet 2(1975)69-72.
21. REES, R. J. W., PEARSON, J. M. H. and WATERS, M. F. R. Experimental and clinical studies on rifampicin in treatment of leprosy. Br. Med. J. 1(1970)89-92.
22. SHEPARD, C. C. Combinations of drugs against Mycobacterium leprae studied in mice. Int. J. Lepr. 40(1972)33-39.
23. SHEPARD, C. C. Recent developments in the chemotherapy and chcmoprophylaxis of leprosy. Leprologica (Argent) 19 (1974) 230.
24. SHEPARD, C. C. Combinations involving dapsonc, rifampin, clofazimine, and ethionamide in the treatment of M. leprae infections in mice. Int. J. Lepr. 44 (1976) 135-139.
25. SHEPARD, C. C. A brief review of experiences with short-term clinical trials monitored by mouse-foot pad inoculation. Lepr. Rev. 52(1981)299-308.
26. SHEPARD, C. C, LEVY, L. and FASAL, P. Rapid bactericidal effect of rifampicin on M. leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
27. SHEPARD, C. C, LEVY, L. and FASAL, P. Further experience with the rapid bactericidal effect of rifampin on Mycobacterium leprae. Am. J. Trop. Med. Hyg. 23(1974)1120-1124.
28. TOMIOKA, H. and SAITO, H. Therapeutic efficacy of some new quinolones and a combination of ofloxacin with rifampin against Mycobacterium leprae infection induced in athymic nude mice. Int. J. Lepr. 61(1993)250-254.
29. XIONG, J-H., Ji, B., PERANI, E. G., PETINON, C. and GROSSET, J.-H. Further study of the effectiveness of single doses of clarithromycin and minocycline against Mycobacterium leprae in mice. Int. J. Lepr. 62(1994)37-42.
1. M.D.; San Francisco Regional Hansen's Disease Program, San Francisco, California, U.S.A.
2. San Francisco Regional Hansen's Disease Program, San Francisco, California, U.S.A.
3. M.D.; Gillis W. Long Hansen's Disease Center, Carville, LA 70721, U.S.A.
4. Gillis W. Long Hansen's Disease Center, Carville, LA 70721, U.S.A.
5. M.S., Gillis W. Long Hansen's Disease Center, Carville, LA 70721, U.S.A.
Reprint requests to Robert H. Gelber, M.D., 2211 Post Street, Suite 301, San Francisco, CA 94114, U.S.A.
Received for publication on 26 July 1994.
Accepted for publication in revised form on 28 November 1994.