- Volume 63 , Number 2
- Page: 202–12
Treatment ot bacilliferous BL/LL cases with combined chemotherapy and immunotherapy
ABSTRACT
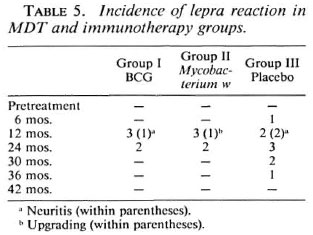
Thirty-six, untreated borderline Iepromatous/lepromatous (BL/LL) leprosy patients with an initial bacterial index (BI) of 4+ to 6+ were serially allocated to three treatment groups. Group I patients received a slightly modified WH O regimen (rifampin once a month, clofazimine and dapsone daily) and BCG intradermally (i.d.) (0.1 mg/ per dose). Group II patients were administered the same MDT and Mycobacterium w (2 x 108) killed bacilli/dose i.d., and Group III received the same MDT with 0.1 ml of distilled water i.d. Vaccination was repeated every 6 months. Biopsies were taken f rom the local site of vaccination and f rom a distant site, i.e., the back. The progress was monitored periodically by clinical, histopathological and bacterial (BI, mouse foot pad, ATP) parameters. Twenty-five patients had completed a follow up of more than 2 years. These included: 7 in Group I, 10 in Group II, and 8 in Group III. One patient of the MDT + BCG group who was progressing well dropped out after 28 months. In cases on combined chemotherapy and immunotherapy, no viable bacilli were demonstrable by mouse foot pad and ATP measurement after 6 months (at 12 months or afterward). However, in come of the control cases on MDT alone, viable bacilli could be detected even up to 18 months (by mouse foot pad) and 2 years (by ATP estimation). With 36 months of treatment, the mean BI decreased f rom 4.64+ to 1.66 + in the group on MDT alone (controls), 4.9 + to 0.08+ in the MDT + BCG group, and 4.75+ to 0 in the MDT + Mycobacterium w group. Compared with the MDT and MDT + BCG groups, the fall in the BI was significantly more in the MDT + Mycobacterium u'group at 12, 18, and 24 months. While all of the cases in the Mycobacterium w groups became smear negative by 36 months, it took 42 months for all of the BCG group to achieve negativity. Immunotherapy appears to have a significant effect on the killing and clearance of bacilli and should be considered as an adjunct to chemotherapy, especially in bacilliferous lepromatous cases.RÉSUMÉ
Trente-six patients présentant une lèpre borderline lépromateuse/ léproniateuse (BL/LL) non traitée avec un indice bactérien initial (IB) de 4 à 6 + ont été répartis en trois groupes de traitement. Les patients du groupe I ont un reçe un régime OMS légèrement modifié (rifampicine une fois par mois, clofa/imine et dapsone tous les jours) et le BCG en intradermique (i.d.) (0.1 mg par dose). Les patients du groupe II ont reçu la même PCT et du Mycobacterium w (2 x 108) bacilles tués par dose i.d.), et les patients du groupe III la même PCT avec 0.1 ml d'eau distillée i.d. La vaccination fut répétée tous les six mois. Des biopsies furent prises à partir du site local de vaccination et d'un site plus distant, dans le dos. L'évolution fut surveillée régulièrement sur base du paramètres cliniques, histopathologiques et bactériens (IB, coussinet plantaire de souris, ATP). Vingt-cinq patients ont terminé un suive de plus de deux ans. Ils se répartissaient comme suit: 7 dans le groupe I, 10 dans le groupe II, et 8 dans le groupe III. Un patient du groupe PCT + BCG qui évoluait favorablement sortit de l'étude après 28 mois. Pour les cas de chimiothérapie combinées, aucun bacille viable ne put être démontré, dans le coussinet plantaire de souris après 6 mois et par des mesures d'ATP (à 12 mois ou par la suite). Cependant, pour certains des témoins sous PCT seule, des bacilles viables purent être détectés jusqu'à 18 mois (par coussinet plantaire) et deux ans (par mesure d'ATP). Après 36 mois de traitement, PIB passa de 4.64 + à 1.66 + dans le groupe sous PCT seule (témoins), de 4.9 + à 0.08 + dans le groupe PCT + BCG, et de 4.75 + à 0 dans le groupe PCT + Mycobacterium w. Comparée à celle des groupes PCT seule et PCT + BCG, la chute de l'IB était significativement plus prononcée dans le groupe PCT + Mycobacterium w aux douzième, dixhuitième et vingt-quatrième mois. Alors que tous les cas du groupe Mycobacterium w avaient leurs frottis négatifs au trente-sixième mois, il fallut 42 mois pour que tous les patients du groupe BCG ne soient négatifs. L'immunothérapie semble avoir un effet significatif sur la destruction et l'élimination des bacilles, particulièrement dans les cas lépromateux riches en bacilles.RESUMEN
Treinta y seis pacientes con lepra lepromatosa subpolar/polar (BL/LL) con un índice bacteriano inicial (BI) de 4 a 6 +, se distribuyeron en 3 grupos. Los pacientes del grupo I recibieron el esquema de tratamiento recomendado por la OMS ligeramente modificado (rifampina una ve/, al mes, clofa/imina y dapsona diariamente) y BCG intradérmico (0.1 mg/dosis). Los pacientes del grupo II recibieron además de la PQT/OMS, 2 x 108 Mycobacterium w muertos (i.d.). Los pacientes del grupo II recibieron la PQT/OMS más 0.1 mi de agua (i.d.). La vacunación se repitió cada 6 meses. Después se tomaron biopsias del sitio local de vacunación y de un sitio distante, p. cjm., la espalda. El progreso de la enfermedad se valoró periódicamente por los criterios clínico, histopatológico, y bacteriológico (BI, almohadilla plantar del ratón y ATP). Veinticinco pacientes habían ya completado un tiempo de seguimiento de más de 2 años (7 del grupo I, 10 del grupo II, y 8 del grupo III). Un paciente del grupo PQT + BCG que iba evolucionado bien, desertó después de 28 meses de seguimiento. Los casos que recibieron la PQT combinada con inmunoterapia no mostraron bacilos viables por las técnicas de la almohadilla plantar y del ATP después de 6 meses del tratamiento y permanecieron sin bacilos después de 12 meses de seguimiento. Sin embargo, en algunos de los casos control sujetos sólo a la PQT/OMS, se pudieron detectar bacilos viables incluso a los 18 (almohadilla plantar del ratón) y a los 24 meses (ATP) del seguimiento. Con 36 meses de tratamiento, el lil promedio disminuyó de 4.64+ a 1.66+ en el grupo PQT/OMS (controles), de 4.9+ a 0.08+ en el grupo PQT + BCG, y de 4.75+ a 0 en el grupo PQT + Mycobacterium w. Comparado con los grupos PQT y PQT + BCG, el grupo PQT/ Mycobacterium w mostró una significante caída en su BI a los 12, 18 y 24 meses. Mientras que en el grupo PQT/Mycobacterium w todos los casos fueron baciloscópicamente negativos a los 36 meses, en el grupo PQT/BCG todos los casos alcanzaron la negatividad sólo hasta los 42 meses. La inmunoterapia parece tener un efecto significativo sobre la muerte y eliminación de los bacilos y debería considerarse como un suplemento de la PQT sobre todo en los casos multibacilares.It is well known that the use of multidrug treatment (MDT) has considerably shortened the duration of treatment of leprosy. However even after 2 years of continuous, standard, currently used MDT, bacilliferous borderline lepromatous/Lepromatous (BL/ LL) cases continue to be smear positive and, also, about 9% to 16% of these cases harbor viable bacilli (17,28 30). It has now been observed that an alarming proportion of these bacilliferous cases who had stopped treatment at 2 years relapsed after 8 to 10 years of follow up [2 and Workshop on Chemotherapy of Leprosy: 14 International Leprosy Congress, Orlando. Int. J. Lepr. 61 Suppl. (1993) 729-730]. In these cases, the dead bacillary skeleton persists for a long period leading to immunological complications such as recurrent lepra reactions. The problem of persistence of dead as well as live bacilli is considered to be due to a partial or complete lack of cell-mediated immune response in lepromatous cases (3). Attempts are being made to alter this ancrgy by using different immunomodulators, including mycobacteria such as BCGand a rapidly growing mycobacterium, Mycobacterium w (13, 31, 32, 34). Immunotherapy may be considered as an alternative approach to achieve faster killing of viable bacilli and clearance of dead bacilli from the body. We had earlier observed promising initial trends of the use of immunotherapy(3). In this communication, we report the results of a long-term follow up (4-5 years) of combined MDT and immunotherapy with BCG or killed Mycobacterium w in untreated bacilliferous BL/LL cases.
PATIENTS AND METHODS
Untreated or less than 3-month treated BL/LL patients with a bacterial index (BI) of 4+ to 6+ on the Ridley scale between the ages of 15 to 60 years were included in the study. The patients were clinically examined in detail and a history of previous drug intake was taken. Patients who had suffered from or were having active tuberculosis, hypertension, diabetes mellitus, or mental illnesses were excluded from the study. The clinical details of the patients included in the study were recorded on a chart; clinical scoring was done by the method of Iyer, et al. (10>). Smears were taken from four sites and an average BI was calculated on Ridley's scale (24).
Thirty-six patients were selected and serially allotted to three treatment groups. All of the patients received modified World Health Organization (WHO) MDT composed of rifampin 600 mg once a month supervised, clofazimine 50 mg daily, and dapsone 100 mg daily unsupervised until the attainment of smear negativity (l5). In addition, the first group (Group I) received BCG (Guindy; 0.1 mg per dose), Group II received Mycobacterium w (supplied by the National Institute of Immunology, New Delhi) in a dose of 2 X 108 killed bacilli per dose, the third group (Group III) was administered distilled water 0.1 ml intradermally (i.d.) as a placebo control every 6 months until the attainment of smear negativity.
Before starting treatment skin biopsies were taken from each patient and subjected to histopathological examination (23,25), mouse foot pad inoculation (7), and bacillary ATP estimation (l7). The allocated vaccine was given to each patient after every 6 months, and induration as well as side effects, if any, were noted after 4 weeks. The size of the nodule was measured, a biopsy was taken from both a local (site of vaccination, forearm) and distant site (area far away from vaccination site, back) and subjected to each of the above tests. The biopsy for histopathology was processed by standard procedures (23,25) A piece of biopsy was homogenized, bacilli (0.03-ml suspension containing up to 104 bacilli) was inoculated into a batch of six randomly bred BALB/C mice. Harvests were done at 1-month intervals after 6 months by the method described by Desikan and Venkataramanaih (7), and a 10-fold increase was taken as positive growth (l8). Another piece of the biopsy was processed for bacillary ATP estimation as per methods described earlier, and the ATP content was calculated as pg/106 bacilli (l7).
All clinical, histological and bacteriological parameters were assessed every 6 months, 4 weeks after each vaccination. The laboratory parameters were all assessed in a single blind manner.
RESULTS
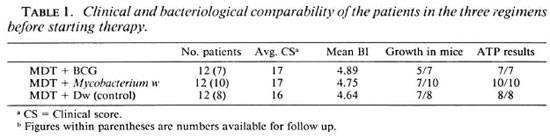
The clinical scores, BI, viability by mouse foot pad, and ATP estimations were analyzed and compared at the start of treatment. It was observed that the patients in the three groups were comparable by all of these parameters (Table 1). Twenty-five of these 36 patients (7 in MDT + BCG, 10 in MDT + Mycobacterium w, and 8 in MDT control groups) were available for follow up for up to 2 years. One patient in the MDT
+ BCG group dropped out after 28 months.
Histopathologically, all of these patients were of BL/LL types with innumerable bacilli, minimal lymphocytes and no epithelioid granuloma or giant cells. The histopathological picture in almost all of the cases was of the LL type showing macrophage granuloma. One case in the BCG treatment group and one in the Mycobacterium w treatment group showed the presence of a good number of lymphocytes and probably belonged cither to the LLs or BL group (23). The detailed observations of histological changes after immunotherapy have been published elsewhere (23).
The observations regarding clinical and bacteriological (viability and BI) progress are summarized below.
MD T + BCG (Group I)
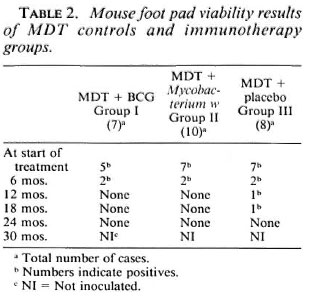
The mean clinical score of the patients in this group was 17; the BI was 4.89. In the pretreatment biopsy specimens 5/7 patients had growth in the mouse foot pad (Table 2), and all of the patients had high levels of bacillary ATP (mean ± S.E.M. = 126.24 ± 33.66 pg/106; Table 4).
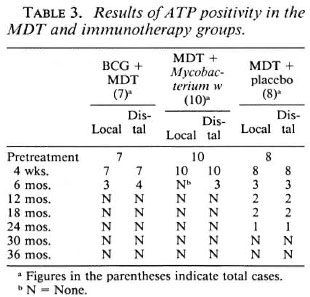
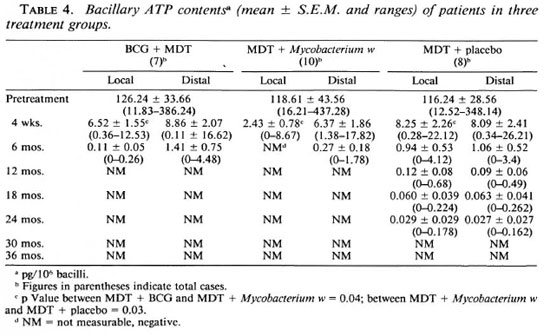
All of the patients showed a positive reaction to vaccination at the local site. The redness and induration was more than 10 mm in diameter and in three patients there was ulceration. No systemic adverse effects of vaccination were observed. At 4 weeks after the first vaccination, the BI remained unchanged. Although there was a drastic reduction in the ATP values, all of the patients had a significant amount of bacillary ATP at both local (mean ± S.E.M.= 6.52 ± 1.55 pg/106) and distal (8.86 ± 2.07 pg/106) sites; Tables 3 and 4).
At 6 months (after the second vaccination), the nodules started to regress and the mean clinical score fell to 12. There was no appreciable fall in the BI (mean BI = 4.7). All of the patients had a local reaction to vaccination in the form of induration and ulceration. However, no systemic adverse reactions were observed. Growth in the mouse foot pad was seen in 2/7 patients. In the bacillary ATP estimations, significant levels were detected in 3/7 at the local site (mean ± S.E.M.= 0.11 ± 0.05 pg/106) and 4/7 (mean ± S.E.M. = 1.41 ± 0.75 pg/106 bacilli) at the distal site (Table 4).
After the third vaccination (12 months), the mean clinical score was 7 and the average BI was 4.07. There was induration at the local site of vaccination in all of the patients. Three patients had a moderate erythema nodosum leprosum (ENL) reaction up to this period, and one had neuritis which was easily controlled. There was no growth in the mouse foot pad and no ATP was detectable in the bacilli isolated from the local as well as the distal sites (Tables 2 and 3).
The patients exhibited good progress at 18 months with a mean BI reaching 2.75 (Table 6) and no viable bacilli detectable by mouse foot pad and ATP estimation (Tables 2 and 3).
By 2 years of treatment, the patients had received five vaccinations; the average clinical score was 2 and the mean BI was 1.36 (Table 6). All patients continued to have a positive local response to vaccination. In two patients there were ENL reactions which subsided with treatment (Table 5). The bacillary count in the biopsies was too low to be inoculated into a mouse foot pad. No ATP was detectable in the bacilli purified from cither the local or distal site.
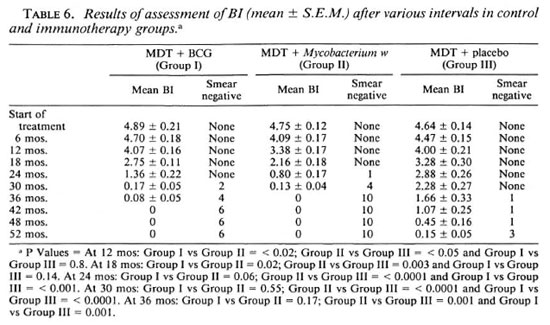
By 2 ½ years, the mean BI was 0.17 and two patients had become skin-smear negative (Table 6). One patient who was clinically and bacteriologically progressing well, as were others in the group, dropped out after 28 months and has not reported back. All of the patients had shrivelling of skin and, therefore, the clinical scores could not be graded further. Because of very low bacillary counts, mouse foot pad inoculation was not possible. No bacillary ATP was detected from local or distal sites.
At the end of 3 years, the mean BI was 0.08 and four patients had become smear negative; others had occasional bacilli at some sites. There was no detectable bacillary ATP and mouse foot pad inoculation could not be done because of very low bacillary counts.
It is noteworthy that all of the patients available for follow up became negative by 42 months. None of these patients had any signs of activity or reactions during the follow up of 6-12 months, and they continue to be smear negative.
MDT + Mycobacterium w (Group II)
The average pretreatment clinical score in the patients in Group II was 17 and the mean BI was 4.75. Seven out of 10 patients showed growth in the mouse foot pad (Table 2), and all 10 patients had high levels of bacillary ATP (mean ± S.E.M. = 118.61 ± 43.56 pg/106 in their biopsies; Tables 3 and 4).
Four weeks after the first vaccination, induration and redness at the local site was recorded in 5/10 patients. None of the patients had any systemic reaction. There was no change in the 131 or clinical scores during this period. All 10 patients had significant bacillary ATP levels at distal sites; whereas marked reduction in bacillary ATP in two cases at the local site was observed. Overall, there was marked reduction in ATP levels with levels of 2.43 ± 0.78 pg/106 at local and 6.37 ± 1.86 pg/106 at distal sites (Tabic 4).
After the second vaccination (6 months), there was erythema and induration at the vaccination site in all patients. However, there was no systemic adverse reaction. Lepromatous nodules had started to regress and the mean clinical score was 12; the average BI was 4.09. Growth in mouse foot pads from two biopsies was observed; the others did not show any growth. No ATP was detectable in biopsies from the local site; measurable levels (mean ± S.E.M. = 0.27 ± 0.18 pg/106) from the distal site were present in 3/10 cases (Tables 3 and 4).
One year after starting therapy (after third injection), the average clinical score was 6 and the mean BI was 3.38 (Table 6). All of the patients showed a positive local response to vaccination. Three patients had ENL reaction, and in one of them there was upgrading of lesions with ulnar neuritis which was easily controlled with steroids (Table 5). There was no growth in mouse foot pads, and no detectable ATP levels in the bacilli from local as well as distal sites from any of the cases.
At 18 months, none of the patients had viable bacilli in biopsies either by ATP estimation or mouse foot pad. The mean BI was 2.16 (Table 6).
Two years after initiating therapy and after having received five vaccinations, the average clinical score was 2 and the mean BI was 0.8. One patient had become smear negative, and two patients had mild ENL reaction in the intervening period (Table 5). Bacillary counts from the specimens were too low to be inoculated into mouse foot pads. No bacillary ATP was detectable in any of the specimens from either of the sites.
At 2 ½ years, the patients' skin showed shrivelling and could not be graded further. The mean BI was 0.13 and four patients had become smear negative. The six patients who were smear positive showed only occasional bacilli in some fields. None of the patients had any reactions. The bacillary counts were too low for mouse foot pad inoculation. ATP was not detectable from any of the specimens from both local as well as distal sites.
All of these patients in therapy Group II became smear negative by 3 years and continued to be negative in 12 months of follow up. There have not been any incidences of reaction and/or relapse during this period.
MD T + distilled water placebo (Group III)
The average pretreatment clinical score was 16 and the BI was 4.64 in Group III. None of the patients had a previous history of lepra reactions. There was growth in 7/8 patients and high bacillary ATP levels (mean ± S.E.M. = 116.24 ± 28.56 pg/106) were present in all specimens (Tables 2, 3 and 4).
Four weeks after the first placebo injection, there were no changes in the clinical scores or Bis. There were no local or systemic reactions. Although there was marked reduction, significant levels of ATP were present in all specimens from the local (mean ± S.E.M. = 8.25 ± 2.26 pg/106) and distal (mean ± S.E.M. = 8.09 ± 2.41 pg/106) sites.
At 6 months (4 weeks after the second injection), the average clinical score was 12, the mean BI was 4.47. Again, there were no local/systemic reactions to the injection; one patient had ENL which was controlled by antireaction treatment. Growth in mouse foot pads from biopsies of two cases was observed at this time. Further, significant ATP levels were detected in three patients from the local (mean ± S.E.M.= 0.94 ± pg/106) and distal (mean ± S.E.M. = 1.06 ± 0.52 pg/106) sites (Tables 3 and 4).
After 1 year of therapy, there were no local responses to the placebo injection. Two patients had ENL with neuritis which was managed with antireaction treatment; the nodules and plaques were regressing; the mean clinical score was 9, and the mean BI was 4.0 (Table 6). There was growth in the mouse foot pad in one patient (Table 2). However, significant ATP levels were observed in two patients at both the local (mean ± S.E.M. = 0.12 ± 0.08 pg/106) and distal (0.09 ± 0.06 pg/106) sites (Table 4).
There was growth in the mouse foot pad in one patient at 18 months (Table 2). ATP was measurable in two patients at 18 months, locally (mean ± S.E.M. = 0.060 ± 0.039 pg/106) and distally (mean ± S.E.M. = 0.063 ± 0.041 pg/106). The bacillary ATP was still detectable in one patient (local 0.224 pg/106, distal 0.262 pg/106) at 24 months. By 2 years, the nodules and plaques had shrivelled, and the average clinical score was 2. The mean BI at this time was 2.88. ENL reaction and neuritis occurred in three patients during this period.
By 2 ½ years of therapy, patients had improved clinically and clinical scores could not be graded further. The mean BI was 2.28. None of the patients had become smear negative. Two of the patients had ENL which was controlled with treatment (Table 5). No growth in the mouse foot pad was observed. All of the specimens were negative for ATP.
After 3 years of treatment, the average BI was 1.66 (Table 6). One patient had ENL which was easily controlled by antireaction treatment, and one patient had become smear negative up to this period. The bacillary counts had decreased further and were too low to be inoculated into mouse foot pads. No ATP was detectable in the bacilli harvested from the tissue biopsies.
By 3 ½ years of therapy, the patients had improved further, and there were no incidences of lepra reactions during this period. The mean BI was 1.07. No patient became negative during this period. Bacillary ATP was not detectable in any of the specimens and, because of very low counts, mouse foot pad inoculation could not be done.
The patients continued to improve at 4 years of therapy. The mean BI was 0.45. No other patients became smear negative. No ATP was detected from bacilli isolated from tissue biopsies from both local and distal sites, and the bacillary count was too low to be inoculated into mice. By 4 ½ years of therapy, the mean BI was 0.15, smears had only occasional bacilli, three patients became smear negative, and none of the patients had any reaction.
DISCUSSION
The use of present day MDT, composed of rifampin, clofazimine and dapsonc, has considerably shortened the duration of treatment in bacilliferous cases to 3-6 years from the life-long treatment advocated in the dapsone monotherapy era. However, further shortening of the duration to 2 years in these bacilliferous patients has been reported to have problems with persisters(17, 28, 30) fairly high relapse rates [2 and Workshop on Chemotherapy of Leprosy, 14 International Leprosy Congress, Orlando. Int. J. Lepr. 61 Suppl. (1993) 729-730], and a large debris of dead bacilli. The incapability to handle these residual problems is perhaps due to a total or partial lack of effective cell-mediated immunity. There have been reports in the literature which show that this unresponsiveness may be altered by various immunological approaches (1, 4, 5, 14, 20, 26, 29, 32, 34) The present pilot trial was planned to investigate the combined immunotherapeutic effect of BCG and Mycobacterium w along with MDT in previously untreated multibacillary patients. After a detailed clinical examination, the patients were serially allocated to three groups. The patients in the three groups were comparable in all parameters. More than two thirds (70%) of the patients were available for follow up for 2 years or more. It was observed that the patients who were regular are also comparable clinically and bacteriologically with the patients who dropped out of the trial. The mean values for these parameters were: Clinical scores (16.4 vs 17.0), BI (4.88 vs 4.56), ATP (all positive in both groups), mouse foot pad positivity rates (75% vs 54%) in regular cases vs dropouts, respectively. Thus, the patients who are available for follow up are representative, and the results are meaningful.
Acceptability and incidence of reaction
In this study it was observed that both BCG and Mycobacterium w were well tolerated. Clinically, the patients in both immunotherapy groups and the MDT controls showed good progress. While there were marginally better responses in the immunotherapy groups, this was not significant. Unlike some of earlier reports (32,34), there was not much difference in the clinical scores in the three treatment groups. Such variation is, perhaps, due to the subjective nature of these parameters and the inability to grade minor differences.
Except for local erythema and ulceration at the site of inoculation, there were no other local or systemic adverse effects from either of the vaccines.
In this study, the immunomodulation did not give rise to an increase in the total ENL or reversal reactions with BCG or Mycobacterium w. As such, the total episodes of ENL reaction and neuritis did not increase with the addition of immunotherapy (Table 5). Rather, ENL reaction continued to occur up to 3 years in patients on MDT alone compared to 2 years for patients on immunotherapy with MDT. The severity of reactions also was not more in patients on immunotherapy. Milder and less frequent reactional (ENL) episodes in patients treated with immunotherapy with Mycobacterium w (33), M. vaccae (29) and live BCG + killed M. leprae (4) have been reported by others also.
Reversal reactions in cases on immunotherapy with Mycobacterium w (12), ICRC (6), and interleukin 2 (11) have been reported. Except for one case with an upgrading reaction in the Mycobacterium w group, there were no reversal reactions in the cases investigated in our study. The difference in the results of Kar, et al. (12) and our study could be due to the selection of cases (all LL except for two LLs/BL in the present study), dose (5 x 108 vs 2 x 108 in the present study), and frequency of vaccination (6 months in the present study compared to 3 months in the study of Kar, et al.). Our observations as well as reported findings by others show that the addition of immunotherapy docs not increase the incidence of type 1 and type 2 reactions in BL/LL cases.
Histological upgrading
Both of the agents used in this study are known immunomodulators. The immunomodulatory potential of BCG alone or in combination for leprosy has been reported by Fernandez (9), Katoch, et al. (14) and Convit, et al. (5). Along with killed M. leprae, BCG has been reported to induce lepromin positivity, histological upgrading and, also, accelerated bacterial clearance (5,21,27). Mycobacterium w has been reported to induce similar changes (12.22,32,34). In the present series of cases, both BCG and Mycobacterium w induced histological changes at local as well distal sites (23). It was observed that a majority of the cases on immunotherapy showed increased lymphocytic infiltration (both at local and distant sites), and some cases showed epithelioid cells as well. Lymphocytic infiltration was slightly more vigorous in those vaccinated with Mycobacterium w. Such changes were not seen in the patients treated with MDT alone. It was also observed that the granuloma fraction reduced much faster in patients in the immunotherapy groups as compared to those on MDT alone. Our observations on the histopathological upgrading induced by Mycobacterium w and other reported observations are similar (32,34). Similar changes also have been reported with the ICRC bacillus (6), M. vaccae (29) and cytokines, such as gamma interferon (20) and interleukin 2 (11). These histological features provide evidence that immunotherapy can positively upgrade immunity in the so-called anergic form of leprosy.
Effect on viability
There have not been any reports on assessment of viability after immunotherapy. The present study provides some insight into the potential microbicidal effect of immunotherapy by using conventional mouse foot pad inoculation (l8) and highly sensitive bacillary ATP estimation of M. leprae (17) recovered from tissue biopsies.
Mouse foot pad results. In all three treatment groups, there was a progressive decline in positive growth in the mouse foot pads. By 1 year or more after starting therapy, there was no growth in the mouse foot pads from tissue biopsies in cither of the immunotherapy groups. In comparison, in patients on MDT + distilled water (controls), growth was observed in one patient from tissue inoculated after 1 year, and this persisted up to 18 months. The trends of positivity rates in the MDT group are like those reported in the THELEP and other studies wherein 9%-15% persister rates were reported by different MDT regimens using thymectomized-irradiated (30) and normal mice (28).
Bacillary ATP measurements. It is known that the normal mouse foot pad method is not a very sensitive method when a few viable bacilli remain in a large pool of dead bacilli, which is the usual case after a few months of treatment with MDT (l9). Using the highly sensitive ATP estimation as an additional measure of viable bacilli, the effect of treatment can be assessed more clearly and also quantitatively (8,16,17). Pretreatment bacillary ATP levels were comparable in all of our treatment groups and were in the same range as reported earlier by us and others (16,17). Significant reduction in the bacillary ATP biomass also occurred as early as 4 weeks with MDT alone (93%) but was more marked in those patients who received MDT + immunotherapy with either BCG (93%-94.8%) or Mycobacterium w (94.7%-98.0%). The fall in the bacillary ATP content was more at the local site compared to the distant site (Table 4). This difference was significant even at 4 weeks in the case of Mycobacterium vvat the local site (p = 0.05, Mycobacterium w vs BCG and Mycobacterium w vs MDT). This effect was more pronounced at 6 months when no ATP could be detected at the local site of vaccination in the MDT + Mycobacterium w group. By 12 months (after the third vaccination), no bacillary ATP was measurable in patients receiving MDT + BCG and MDT + Mycobacterium w, but low levels of bacillary ATP were present in 2/8 cases in the MDT control group and continued to be measurable even at 18 and 24 months (Tables 3 and 4). In an earlier study, bacillary ATP also was detectable in 16% of bacilliferous BL/LL cases treated with the same regimen at the end of 2 years (17).
In our study both vaccines, appear to help in achieving more rapid killing of viable bacilli, as seen by mouse foot pad and bacillary ATP estimations; this effect appears to be more with Mycobacterium w compared to BCG.
Effect on bacillary clearance
While the BI at the start of therapy was comparable in the three therapy groups, the trends in the fall of the BI were different afterward (Tables 1 and 6, Figs. 1 and 2). In the MDT + BCG group (Group I), the fall in BI was significantly more (p <0.001) by 24 months compared to the controls (Group III) (Table 6). Thereafter, the fall in BI was very rapid and by 3 ½ years all of the patients had become smear negative. At 2 ½ years Group I patients achieved approximately a similar mean BI and percentage of smear negativity as was achieved by Group III patients in about 4 ½ years of therapy (15 and present study). Convit, et al. (4) (using a mixture of M. leprae + BCG) and we (using BCG alone14) also have observed accelerated bacterial clearance.
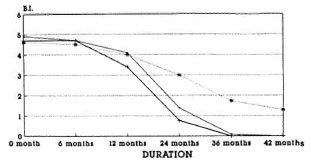
Fig. 1. Fall in the bacterial index (Ridley scale) inthree treatment groups (MDT + BCG = •; MDT +Mycobacterium w = +; MDT + placebo = *).
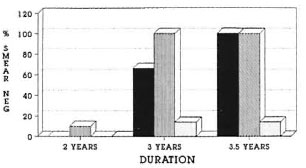
Fig. 2. Proportion of cases (%) achieving smearnegativity in three treatment groups (MDT + BCG =■; MDT + Mycobacterium w =  ; MDT + placebo=
; MDT + placebo=  ).
).
In Group II patients (Mycobacterium w used as immunotherapeutic agent), it was observed that the fall in BI was accelerated quite early. At 1 year, the BI had declined by 1.4 log; this was statistically significant compared to Group III MDT controls (p <0.05) and also Group I MDT + BCG (p <0.02). Thereafter the fall in BI was very rapid, and one patient became smear negative by 2 years of therapy. Compared with BCG groups, the fall in BI was significantly more in the Mycobacterium u'treated group at 12 (p <0.02), 18 (p <0.05), and 24 (p =0.05) months. All patients had become smear negative by 3 years. The observations of Talwar, et al. (32), Zaheer, et al. (34) and Mukherjee, et al. (22), regarding the bacteriological clearance, are similar and indicate the benefit of the addition of immunotherapy with Mycobacterium w. Enhanced bacterial clearance also has been reported with recombinant interleukin 2 (11), gamma interferon (20,24), and the ICRC bacillus (11). Thus, several agents with a potential immunotherapeutic role in the treatment of leprosy are now on the horizon.
The present study, therefore, shows that combined immunotherapy (with cither BCG or Mycobacterium w) and chemotherapy is well tolerated, docs not increase the incidence of reactions, and helps in the faster killing of viable bacilli and clearing of dead bacilli in untreated bacilliferous BL/LL patients. Taken together with histological findings reported earlier (23), the observations of our present study suggest a clear potential immunotherapeutic role for Mycobacterium w and BCG. In our study Mycobacterium w has exhibited better histological (immunological upgrading) and bacteriological (killing as well as clearance) responses. However, such evaluation needs to be carried out in a larger number of cases.
Acknowledgment. The authors are thankful to S. K. Bhan, Shrec Ram, A. Robi, Noel S. Singh, the late Mr. R. P. Agarwal, Mr. Ishat Ali and Noel Crispin, and Mr. Ram Murti for technical and clinical assistance, respectively. The help of Mr. Hari Om Agarwal and Mr. N. Dubcy in preparing the illustrations, Mr. J. D. Kushwah in preparing the manuscript, and the gift of some reagents from LEPRA, U.K., is gratefully acknowledged.
REFERENCES
1. BHATKI, W. S. and CHULAWALA, R. G. The immunotherapeutic potential of ICRC vaccine: a case control study. Lepr. Rev. 63(1992)358-363.
2. BLANC, L., JAMET, P., FAYE, O., SOW, S. and BASKIN, P. Short term rifampin-containing regimens for MB leprosy yield to high risk of relapse. (Abstract) Int. J. Lepr. 61 Suppl.(1993)13A.
3. BLOOM, B. R. and MEHRA, V. Immunological unresponsiveness in leprosy. Immunol. Rev. 80(1994)5-26.
4. CONVIT, J., ARANZAZU, N., ULRICH, M., PINARDI, M. E, REYES, O. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and in Mitsuda-negativecontacts. Int. J.Lepr.50(1982)415-424.
5. CONVIT, J., PINARDI, M. E., RODGIRUEZ, O. G., ULRICH, M., AVILA, J. L. and GOIHMAN-YAHR, M. Elimination of Mycobacterium leprae subsequent to local in vitro activation of macrophages in lepromatous leprosy by other mycobacteria. Clin. Exp. Immunol. 17(1974)821-826.
6. DEO, M. G., BAPAT, C. V., BIIALERO, V., CHATUR-VEDI, R. M., BHATKI, W. S. and CHULAWALA, R. G. Antileprosy potential of ICRC vaccine: a study in patients and health volunteers. Int. J. Lepr. 51(1983)540-549.
7. DESIKAN, R. V. and VENKITARAMANAIAH, H. N. A modified method of harvesting M. leprae from foot pads of mice. Lepr. India 48(1976)157-162.
8. DHOPLE, A. M. Application of ATP assay to patient care in leprosy. In: XII International Leprosy Congress Proceedings, New Delhi, February 20-25,1984. Desikan, K. V., ed. New Delhi: Printaid, n.d., pp. 358-360.
9. FERNANDEZ, J. M. M. Use of BCG in immunoprophylaxis of leprosy. Rev. Argent. Dermatol. 23(1939)425.
10. IYER, C. G. S., BALAKRISHNAN, S. and RAMU, G. A comparison of low and conventional dosage of dapsone in the treatment of lepromatous leprosy. Lepr. India 49(1977)372-386.
11. KAPLAN, G., BRITTON, W. J., HANCOCK, G. E., THEUVENET, W. J., SMITH, K. A., JOB, C. K., ROCHE, P. W., MOLLOY, A., BURKHARDT, R., BARKER, J., PRADHAN, H. M. and COHN, Z. A. The systemic effect of recombinant interleukin 2 on the manifestations of lepromatous leprosy. J. Exp. Med. 173(1991) 993-1006.
12. KAR, H. K., SHARMA, A. K., MISRA, R. S., BEENA, K. R., ZAHEER, S. A., MUKHERJEE, R., MUKHERJEE, A., PARIDA, S. K., WALIA, R., NAIR, S. K. and TALWAR, G. P. Reversal reaction in multibacillary leprosy patients following MDT with and without immunotherapy with a candidate antileprosy vaccine, Mycobacterium w. Lepr. Rev. 64(1993)219-226.
13. KATOCH, K., NATARAJAN, M., BAGGA, A., KATOCH, V. M., SREEVATSA, SHARMA, V. D . and Sun VANNAVAR, C. T. Immuno-therapeulic trials using BCG and Mycobacterium strain w along with MDT in highly bacillated BL /LL cases. Quad. Coop. Sanit 12(1992)239-244.
14. KATOCH, K., NATARAJAN, M., NARAYANAN, R. B. and KATOCH, V. M. Immunotherapy of treated BL/LL cases with BCG: histological, immunohistological and bacteriological assessment. Acta Leprol. 7 Suppl. 1(1989) 153-155.
15. KATOCH, K., RAMU, G., RAMANATHAN, U., SREEVATSA, SENGUPTA, U., SHARMA, V. D., SHIVANNAVAR, C. T. and KATOCH, V. M. Results of a modified WHO regimen in highly bacilliferous BL /LL patients. Int. J. Lepr. 57(1989)451-457.
16. KATOCH, V. M. Application of bioluminescent technology to patient care and research in leprosy. (Editorial) Indian J. Lepr. 61(1989)313-322.
17. KATOCH, V. M., KATOCH, K., RAMANATHAN, U., SHARMA, V. D., SHIVANNAVAR, C. T., DATTA, A. K. and BHARADWAJ, V. P. Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining. Int. J. Lepr. 57(1989)615-621.
18. LEVY, L. Multiplication of Mycobacterium leprae in normal mice. Int. J. Lepr. 55 Suppl. (1987)615-621.
19. LEVY, L. Application of mouse foot pad technique in immunologically normal mice in support of clinical drug trials and a review of earlier drug trials in lepromatous leprosy. Int. J. Lepr. 55 Suppl. (987) 823-828.
20. MATHUR, N. K., MITTAL, A., MATHUR, D. and JAIN, S. K. Long term follow up of lepromatous leprosy patients receiving intralcsional recombinant gamma interferon. Int. J. Lepr. 60(1992)98-100.
21. MEYERS, W. M., MCDOUGALL, A. C, FLEURY, R. N., NEVES, R., REYES, O. and BINFORD, C. H. Histologic responses in sixty multibacillary leprosy patients inoculated with autoclaved Mycobacterium leprae and live BCG. Int. J. Lepr. 56(1988)302-309.
22. MUKHERJEE, A., ZAHEER, S. A., SHARMA, A. K., MISRA, R. S., KAR, H. K., MUKHERJEE, R. and TALWAR, G. P. Histopathological monitoring of immunotherapeutic trial with Mycobacterium w. Int. J. Lepr. 60(1992)28-35.
23. NATRAJAN, M., KATOCH, K., BAGGA, A. K. and KATOCH, V. M. Histological changes with combined chemotherapy and immunotherapy in highly bacillated lepromatous leprosy. Acta Leprol. 8(1992)79-86.
24. RIDLEY, D. S. Bacterial indices. In: Leprosy in Theory and Practice. Cochrane, R. G. and Davey, T. F., eds. Bristol: John Wright and Sons Ltd., 1964, pp.620-622.
25. RIDLEY, D . S. Histological classification and the immunological spectrum of leprosy. Bull. WHO 51(1974)451-464.
26. SAMUEL, N. M., GRANGE, J. M., SAMUEL, S., LUCAS, S., OWILLI, C. O. W., ADALLA, S., LEIGH, J. M. and NAVARETTE, C. A study of effect of intradermal administration of recombinant gamma interferon in lepromatous leprosy patients. Lepr. Rev. 58(1987)389-396.
27. SAMUEL, N. M., NEUPANI, K., LOUDON, J. and SAMUEL, L. S. Vaccination of leprosy patients and healthy contacts. Indian J. Lepr. 57(1965)288-296.
28. SREEVATSA, GIRDHAR, B. K. and DESIKAN, K. V. Screening of drug resistant strains of Mycobacterium leprae in lepromatous leprosy patients under multidrug treatment. Indian J. Med. Res. 87(1988)139-143.
29. STANFORD, J. L., ROOK, G. A. W., BAIIR, G. M., DOWLATTI, Y., GANAPATI, R., GHAZI SAIDI, K., LUCAS, S., RAMU, G., TORRES, P., LY, H. M. and AUSTEY, N. Mycobacterium vaccae in the immunoprophylaxis and immunotherapy of leprosy and tuberculosis. Vaccine 8(1990)525-530.
30. SUBCOMMITTEE ON CLINICAL TRIALS OF THE SCIENTIFIC WORKING GROUP OF CHEMOTHERAPY OF LEPROSY (THELEP) OF UNDP/WORLD BANK/ WHO SPECIAL PROGRAMME IN TROPICAL DISEASES. THELEP controlled drug trials. Int. J. Lepr. 55 Suppl.(1987)864-868.
31. TALWAR, G. P. and FOTEDAR, A. Two candidate antiLeprosy vaccines-current status of their development. Int. J. Lepr. 51(1983)550-553.
32. TALWAR, G. P., ZAHEER, S. A., MUKHERJEE, R., WALIA, R., MISRA, R. S., SHARMA, A. K., MUKHERJEE, A., PARIDA, S. K., SURESH, N. R., NAIR, S. K. and PANDEY, R. H. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable Mycobacterium w in multibacillary patients. Vaccine 8(1990)121-129.
33. ZAHEER, S. A., MISRA, R. S., SHARMA, A. K., BEENA, K. R., KAR, H. K., MUKHERJEE, A., WALIA, R. and TALWAR, G. P. Immunotherapy with Mycobacterium w vaccine decreases the incidence and severity of type 2 (ENL) reactions. Lepr. Rev. 64(1993)7-14.
34. ZAHEER, S. A., MUKHERJEE, R., BEENA, K. R., MISRA, R. S., SHARMA, A. K., KAR, H. K., KAUR, H., NAIR, S. K., MUKHERJEE, A. and TALWAR, G. P. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J. Infect. Dis. 167(1993)401-410.
1. M.D., Assistant Director, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
2. M.D., Deputy Director, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
3. M.B.B.S., D.V.D., Senior Research Officer, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
4. M.Stat., Assistant Director, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
5. Ph.D., Senior Research Officer, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
6. Ph.D., Senior Research Officer, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
7. Ph.D., Research Officer, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
8. Ph.D., Research Assistant, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
9. Ph.D., Research Assistant, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
10. Ph.D., Officerin-Charge, Central JALMA Institute for Leprosy (ICMR), Tajganj, Agra 282001, India.
Present address for Dr. Sreevatsa: OIL Field Unit, Avadi, Madras, India. Present address for Dr. Patil: Krishna Institute of Medical Sciences, Karad, Maharastra, India.
Received for publication on 4 August 1994.
Accepted for publication in revised form on 14 December 1994.