- Volume 63 , Number 2
- Page: 222–30
Clinical and histological discrepancies in diagnosis of ENL reactions classified by assessment of acute phase proteins SAA and CRP1
ABSTRACT
Sixteen out of 45 (36%) leprosy patients with clinical features of acute erythema nodosum leprosum (ENL) did not show the characteristic presence of neutrophils (polymorphs) in histology of the ENL lesion. The acute-phase reactants, serum amyloid A (SAA) and C-reactive protein (CRP) which are systemic markers of inflammation, and IgM and IgG antibody to Mycobacterium leprae were determined in these patients in order to understand the differences in histological diagnosis. Both SAA and CRP were elevated in ENL patients, irrespective of the presence of polymorph infiltrates, as compared to nonreactional lepromatous patients, patients with histologically confirmed reversal reactions and endemic controls, indicating that all clinically diagnosed ENL patients had ongoing inflammatory reactions. On the other hand, IgM and IgG antibodies were significantly lower (> 70%) in ENL patients as compared to nonreactional lepromatous patients. When the two ENL groups [ENL-PMN + ve (positive for neutrophils) and ENL-PMN -ve (negative for neutrophils)] were compared, there were no significant differences in the mean SAA, IgM or IgG antibody concentrations, but CRP was eightfold lower in ENL-PMN-ve as compared to the ENL-PMN+ve group. This may indicate that the timing or modulation of the reaction was different in the two ENL groups. Thus, measurement of the acute-phase response and the ratio of SAA/ CRP in particular are helpful in the clinical diagnosis of ENL reactions in leprosy.RÉSUMÉ
Seize parmi 45 (36%) malades de la lèpre présentant les signes cliniques d'un érythème moucux lépreux (ENL) aigu ne montraient pas la présence caractéristique de neutrophiles (polymorphonucleaires) à l'histologie des lésions d'ENL. On a déterminé chez, ces patients les réactifs de la phase aiguë, l'amyloide A sérique (SAA) et la protéine C-réaclive (CRP), qui sont des marqueurs systémiques de l'inflammation, et les anticops IgM et IgG vis-à-vis de Mycobacterium leprae afin de comprendre les différences dans le diagnostic histologique. Le SAA et la CRP étaient tous deux élevés chez les patients ENL, indépendemment de la présence d'infiltrats de polymorphonucleaires, en comparison avec des patients lépromateux qui n'étaient pas en réaction, des patients avec des réactions réverses histologiquement confirmées et des témoins de régions endémiques, indiquant que tous les patients avec un ENL cliniquement diagnostiqué avaient des réactions inflammatoires en cours. D'un autre côté, les anticorps IgM et IgG étaient significadvement plus bas (> 70%) chez les patients avec ENL que chez les témoins lépromateux non en réaction. Quand les deux groupes ENL [ENL/PMN+ (positifs pour les neutrophiles) et ENL/PMN- (négatifs pour les neutrophiles)] étaient comparés, il n'y avit pas de différence significative dans le SAA moyen, les concentrations d'anticorps IgM ou IgG, mais la CRP était huit fois plus basse dans le groupe ENL/PMN- par rapport au groupc ENL/ PMN + . Ccci pourrait indiqucr que revolution dans le temps ou la modulationn de la reaction étaicnt differentes dans les deux groupes ENL. En consequence, la mesure de la réponsc de la phase aigué et le ratio SAA/ CRP en particulier sont une aide au diagnostic clinique des reactions d'ENL dans la lépre.RESUMEN
Diez y seis de 45 (36%) pacientes con lepra en reacción tipo eritema nodoso leproso (ENL) carecieron de la característica presencia de neutrófilos (polimorfos) en los cortes histológicos de la lesión. Con el fin de entender las diferencias en cl diagnóstico histológico de las lesiones, se determinaron los niveles de dos reactantes de fase aguda (amiloide A del suero, SAA, y proteína C-reactiva, CRP), así como los niveles de anticuerpos IgM e IgG contra Mycobacterium leprac. A diferencia de lo observado en los pacientes lepromatosos no reaccionales, en los pacientes en reacción reversa histológicamente confirmada y en los controles de zona endémica, los pacientes con ENL tuvieron niveles elevados tanto de SAA como de CRP, y esto fue independiente de la presencia de polimorfos infiltrantes en sus lesiones. Lo anterior fue indicativo de que todos los pacientes con ENL estudiados, presentaban una reacción inflamatoria. Por otro lado, los niveles de anticuerpos IgM e IgG estuvieron significativamente más bajos (>70%) en los pacientes con ENL que en los pacientes lepromatosos no reaccionales. Cuando se compararon los dos grupos de pacientes con ENL (ENL-PMN negativos y ENL-PMN positivos) no se encontraron diferencias en las concentraciones de SAA y de los anticuerpos IgG o IgM, pero los niveles de CRP fueron 8 veces mayores en los pacientes ENL-PMN positivos que en los pacientes ENL-PMN negativos. Esto podría indicar que el tiempo de evolución o la modulación de la reacción en el momento del estudio fue diferente en los dos grupos de pacientes. Así, la medición de la respuesta de fase aguda y la relación SSA/CRPcn particular, pueden ser de utilidad en el diagnostico clínico de las reacciones ENL en la lepra.Reactions in patients with leprosy are the most serious complications of the disease and must be diagnosed early and treated effectively if permanent disability is to be avoided. In multibacillary or lepromatous leprosy, systemic complications may occur suddenly during reactional episodes which are termed erythema nodosum leprosum (ENL). Clinically, ENL (10) is characterized by recurrent crops of painful, red, indurated, subcutaneous nodules arising in apparently normal skin which may become necrotic, pustular and hemorrhagic. Patients with severe ENL can develop fever, lymphadenopathy, albuminuria and arthritis as well as iridocyclitis, orchitis and neuritis. Histological examination of early ENL lesions reveals a predominantly polymorphonuclear leukocyte inflammatory reaction within pre-existing lepromatous lesions, often associated with vasculitis (10). Most of the clinical and histological features associated with ENL resemble serum sickness (30). The demonstration of immune complexes in serum (2,20) and in skin (24,30) adds weight to the immune complex pathogenesis of ENL. However, some investigators (9, 13) have shown evidence for a primary T-lymphocyte pathogenesis of the condition, and it remains unclear whether the neutrophil leukocytes precede the chemotaxis of lymphocytes into the lesions. Nearly 50% of lepromatous leprosy patients develop ENL by the end of their first year of chemotherapy (30). The ENL reaction is viewed as a serious event which, in the past, necessitated the temporary discontinuation of leprosy chemotherapy while antiinflammatory agents, including steroids and thalidomide, were instituted (16,23). ENL episodes also can occur in patients in whom chemotherapy has not been initiated, indicating that ENL is not just a complication of treatment but is more likely a consequence of lepromatous leprosy itself (l9). The factors which precipitate ENL reactions in lepromatous leprosy patients are still speculative.
Patients with lepromatous leprosy have high concentrations of acid-fast bacilli (AFB) in tissues and antibodies of both IgM and IgG isotypes in the circulation (15). These antibodies are believed to play little or no role in protection from leprosy but may be important in the pathogenesis of ENL. In these patients, ENL has been shown to be associated with a diminution of bacterial load associated with rapid antigen clearance (22). Activation of the complement pathway by complement fixing antibodies and the concomitant release of inflammatory mediators may then cause the pathogenesis and clinical symptoms associated with ENL episodes.
This study sought to identify markers of ENL reactions in both the skin and peripheral blood of patients presenting with acute clinical signs of ENL. Serum concentrations of the acute phase reactants serum amyloid A (SAA) and C-reactive protein (CRP) have been shown to increase 100-1000-fold in the 24-48 hr following an inflammatory stimulus(18),and SAA has been shown to be elevated early in ENL reactions (11). In addition to histology we measured the acute phase proteins to evaluate their usefulness in the diagnosis of ENL.
MATERIALS AND METHODS
Patient material. The study group consisted of 45 histologically confirmed leprosy patients with clinical features of ENL, including tender papules and nodules, and fever. Other symptoms recorded were arthritis (73%), neuritis (55%) and iritis (3.7%). At the time of biopsy, 19 were newly diagnosed patients, 4 were on dapsonc monotherapy, and 20 were receiving dapsone and clofazamine. None of the patients with ENL were on thalidomide and only one was receiving steroid treatment.
A 4-mm punch biopsy was taken from a representative skin lesion and fixed by conventional formol-mercuric chloride-acetic acid (FMA) fixative, processed to paraffin and stained with hematoxylin and eosin (H + E) and Wade-Fite stains for AFB. Leprosy lesions were categorized using standard clinical and histopathological features (25,26). Typical ENL was recognized as the classical "pink node" type (25), with a polymorphonuclear neutrophil (PMN) infiltrate, with/ without vasculitis, into the dermis and subcutis of lepromatous leprosy lesions.
Control groups included: untreated leprosy patients (N = 23), classified as lepromatous (LL) or borderline lepromatous (BL); 14 leprosy patients clinically and histologically undergoing reversal reactions (type 1), and 42 healthy endemic controls, without known contact with leprosy, who were employed at the Aga Khan University Medical Center. Five to 10 ml of blood was collected in veneject tubes without anticoagulant. Blood was kept at room temperature for 1- 2 hr and then overnight at 4ºC. Serum was separated, centrifuged to remove red blood cells, and then further aliquotted in small volumes and stored at -70ºC.
Antibodies. Mouse monoclonal antibodies against human C-reactive proteins were a gift from Dr. Potempa, Chicago, U.S.A., to Dr. McAdam. Monoclonal antibodies against human SAA were obtained from Dako, and against serum amyloid P (SAP) were generated by G. O. Sullivan and J. Raynes at the London School of Hygiene and Tropical Medicine. Rabbit polyclonal antibodies to CRP were affinity-purified with human CRP and absorbed with normal human serum before use. Goat antihuman SAA was obtained by a similar procedure.
Quantitation of acute phase proteins. Serum amyloid A protein (SAA) determinations were carried as described (21) using a competitive inhibition ELISA with a goat anti-human SAA and an SAA-alkaline phosphatase conjugate; the ability of samples to inhibit conjugate binding was compared with that of the standards (bovine high density lipoprotein (HDL) and a known SAA concentration as described in detail earlier (21). To measure CRP a similar assay was used, coating the plates with affinitypurified rabbit antibodies to human CRP (5).
Assessment of IgM and IgG anti-MLSON antibodies. ELISA were performed as described in detail previously (7,8). Mycobacterium leprae soluble sonicate (MLSON) batch CD 114 was kindly provided by Dr. R. J. W. Rees, National Institute for Medical Research, Mill Hill, London, U.K. Units of activity were assigned to the test serum by comparison to reference sera: the activity assigned to the IgM reference serum was 6400, to the IgG reference serum 51,200 units with one unit giving an optical density reading twice above the negative control.
Statistical analysis. Analyses, including Fisher's exact test and Mann-Whitney analysis, were performed on a Macintosh microcomputer using a Statview™ package.
RESULTS
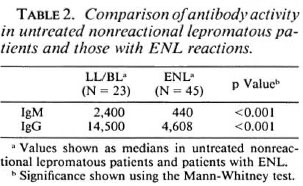
Characteristics of ENL patients. Fortyfive patients were recruited who fulfilled the clinical criteria of acute ENL. The mean age of the ENL patients was 32 years (range 17- 71 years), with a male : female ratio of 3:1. The mean bacterial index (BI) in skin lesions was 3.76 ± 0.2 (S.E.M.). The majority of patients (40/45) developing ENL had lepromatous (LL) leprosy; the remaining 5 patients had borderline lepromatous (BL) leprosy. Sixty-four percent (29/45) of the patients with clinical ENL had typical ENL on histology, including polymorph infiltrate (ENL-PMN+ve), but 16/45 (36%) had no acute polymorph infiltrate (ENL-PMN- ve) superimposed on the lepromatous leprosy (Fig. 1). The ENL-PMN-ve biopsies did not show necrosis or the variant, connective tissue pattern of ENL ( 25,27). No significant difference was observed between the two groups with respect to temperature, neuritis, arthritis or delay in diagnosis (results not shown). Acute phase reactants such as serum amyloid A (SAA) and C-reactive protein (CRP) are usually elevated in the initial phase of an inflammatory response. To investigate if polymorph infiltrate is associated with the early phase of the response, we measured the quantitative levels of these acute phase proteins in ENL patients with and without polymorph infiltrates to investigate if these differences were associated with variable levels of other markers of acute inflammation.
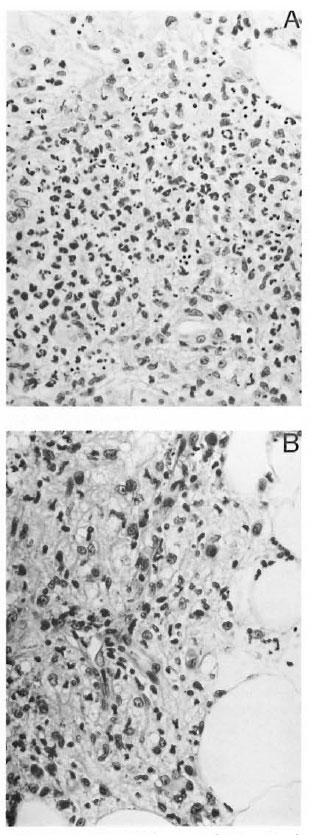
Fig. 1. A = ENL-PMN+ ye, showing deep dermis with foamy macrophages (lepromatous leprosy) infiltrated by numerous polymorphonuclear leukocytes(H&E x400). B = ENL-PMN-ve, showing deep dermis with foamy macrophages and some lymphocytes,but no polymorphonuclear leukocyte infiltrate (H&Ex 500).
Concentrations of acute phase proteins in ENL. SAA and CRP were measured in both the histologically ENL-PMN + ve and ENL-PMN- ve groups of patients, in patients with multibacillary leprosy (LL/BL) but without any clinical or histological signs of reaction, leprosy patients undergoing reversal reactions (RR), and in a group of healthy endemic controls (EC). SAA concentrations were comparable in ENL-PMN + ve and ENL-PMN-ve groups and significantly elevated (p < 0.001) as compared to LL/BL patients without any clinical or histological evidence of ENL and the healthy endemic control group (Fig. 2). Patients in reversal reaction also did not show any elevation of SAA, and the group median was comparable to LL/BL (Fig. 2). These results indicate that the PMN-ve group was also distinct from patients with RR reactions in terms of acute phase proteins. The majority of patients with clinical signs of ENL, with or without polymorph infiltrate, therefore had an ongoing inflammatory response. It was interesting to note that two LL/BL patients with the highest SAA developed an ENL reaction within the next 2 weeks, indicating that SAA may be an early predictor of ENL.
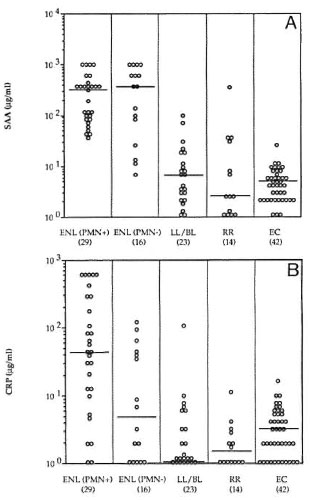
Fig. 2. A = Serum amyloid A (SAA) and B =C-reactive protein (CRP) concentrations in leprosy patients with clinical symptoms of acute ENL reactionswho were reported histologically positive (ENL-PMN+ve) or histologically negative (ENL-PMN-ve);untreated lepromatous leprosy (LL/BL); reversal reactions (RR); and endemic controls (EC). Horizontallines show medians for each group.
CRP also was raised in the majority of ENL-PMN+ve (median 45 μg/ml) group but a bimodal distribution for CRP was observed in the ENL-PMN-ve group with 10/ 16 showing very low CRP (< 5 μg/ml) and 6/16 showing concentrations similar to ENL-PMN + ve group (> 30 μg/ml). None of the other groups including LL/BL, RR, and EC showed significant levels of CRP.
Table 1 summarizes the information in Figure 1 with the statistical analysis for the ENL-PMN + ve and ENL-PMN-ve groups. There were no significant differences in the mean concentrations of SAA in ENLPMN+ve and ENL-PMN-ve patients; however, the mean concentrations of CRP were significantly lower in the group with an absence of polymorph infiltrate (p < 0.001). This difference was even more striking when the SAA/CRP ratio was calculated for individual patients. The mean ratio in ENL-PMN+ve patients was fourfold higher than the mean ratio in ENL-PMN-ve patients (p < 0.001). ENL is commonly described as an antibody-mediated pathological process initiated by immune complex deposition in the tissues with activation of the inflammatory process due to complement activation. It was therefore possible that CRP was binding to the components of mycobacteria or complement in immune complexes which may be depositing in the tissues during ENL. We have, therefore, compared antibody concentrations to MLSON in all groups of patients to see if there were differences in antibody concentrations as well.
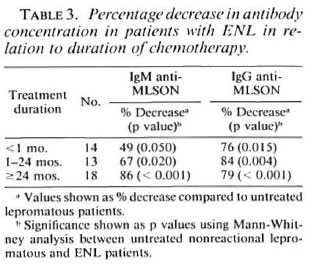
Antibody concentrations in ENL. Figure 3 shows the distribution of IgM and IgG antibodies in lepromatous leprosy patients with and without ENL. The majority of patients developing ENL had LL leprosy. Antibody for both isotypes was > 70% lower (p < 0.001) in the ENL group compared to the nonreactional lepromatous patients (Table 2), suggesting that immunoglobulins may be depositing in the tissues. Since many patients with ENL had been on chemotherapy, an overall decrease of antibody concentration in serum might alternatively be associated with antigen clearance during therapy (3). As shown in Table 3, antibody concentrations for both isotypes decreased in ENL with the length of chemotherapy and was more marked for IgG than for IgM. However, the antibody concentration was still significantly lower for both IgM (p < 0.05) and IgG (p < 0.01) in patients who presented with ENL within a month of the initiation of chemotherapy compared to untreated lepromatous leprosy patients without ENL reaction, supporting immune complex deposition in tissues of ENL patients. However, there was no significant difference in the antibody concentrations in the ENL-PMN + ve and ENL-PMN-ve groups, indicating that the difference in CRP in these two ENL groups is not related to differences in the amounts of immune complexes and bound CRP being deposited in the tissues.
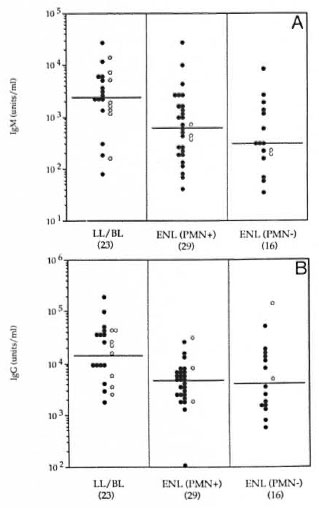
Fig. 3. Concentrations of antibodies to NILSON(A = IgM; B = IgG) in leprosy patients with untreatedlepromatous leprosy without reaction (LL/BL); pa-tients with ENL-PMN+ve and patients with ENL-PM N - ve. ● = LL patients; ○= BL patients; horizontallines show medians for each group.
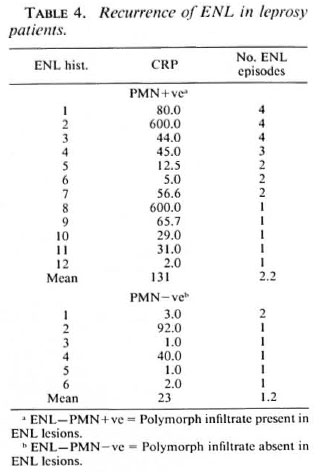
As part of a larger study of the incidence of reactions in newly diagnosed patients (manuscript in preparation), a subset ofENL patients (N = 18) were checked for recurrence of ENL by reviewing the records and the findings are summarized in Table 4. The follow-up period after the occurrence of the first ENL episode was a minimum of 2 years. The ENL-PMN + ve group showed a much higher incidence of recurring ENL reactions (7/12) than did the ENL-PMN-ve group (1 /6). The number of recurrent episodes were also higher in the PMN + ve group compared to the PMN-ve group where only one patient showed a second event of ENL (and the records indicated that it was a mild episode not requiring hospital admission). When Fisher's exact test was applied to the two groups the difference in recurrence was not significant, probably due to the small number in the PMN-ve group. These findings suggest that ENL patients with polymorph infiltrate and high levels of CRP are at a higher risk of recurrence of ENL and this issue warrants further study.
DISCUSSION
Clinically, ENL reactions in leprosy patients are more readily identifiable than other reactions, such as reversal (upgrading, downgrading), or exacerbation of disease, although some overlap may occur. It was, therefore, surprising when 35.5% of our clinically diagnosed ENL patients, despite classical clinical signs and symptoms of ENL, showed an absence of polymorph infiltration which is considered a hallmark of ENL histology. No significant difference was observed between the two groups with respect to temperature, neuritis and arthritis. Care was taken to exclude factors contributing to misdiagnosis, such as biopsy from a nonreactional or postreactional site. The histology of ENL changes over time with loss of the acute inflammatory component (l0), but equivalent majorities of both ENLPMN+ve and ENL-PMN-ve groups of patients were biopsied within 7 days of onset of clinical ENL. Since ENL is essentially an acute inflammatory episode, we assessed the acute phase reactants SAA and CRP which have been shown to correlate with the degree of inflammation and severity of disease in inflammatory conditions such as arthritis and systemic lupus erythematosus (18). In an earlier report, elevated levels of SAA were shown to occur in 15%-30% of patients across the leprosy spectrum with a higher incidence toward the lepromatous pole (11). Our results show that the frequency of raised SAA reaches 100% in patients with clinical signs and symptoms of ENL irrespective of the presence of polymorph infiltrates in the tissue. Elevated SAA concentrations may be the earliest indicator of the onset of ENL as seen in 2/23 lepromatous patients who showed the highest SAA concentrations and later developed ENL (within 2 weeks of serum sampling). However CRP concentrations in our ENLPMN+ve patients were much higher than in ENL-PMN-ve patients, indicating that there may be a difference in the ENL reaction occurring in the two groups. This was particularly striking when the ratio of SAA/ CRP was compared in ENL-PMN+ ve and ENL-PMN-ve patients. One possible explanation may be that the two groups are at different stages of evolution of the reaction. Just as the early ENL skin lesions are characterized by a predominance of polymorphonuclear leukocytes and the later stage by lymphocytes, similarly the dynamics of SAA and CRP may also be different with CRP being downregulated earlier than SAA.
Acute phase proteins are synthesized in the liver in response to inflammatory cytokines such as IL-1 and IL-6 (14,28); they may be better markers of inflammation than IL-1 and IL-6 in that they represent amplified readouts of the cytokine response which are less variable over time. In most inflammatory conditions, a high overall correlation between SAA and CRP has been reported (29), although SAA and CRP can be differentially regulated; IL-1 is more stimulatory of SAA (25); whereas IL-6 favors CRP synthesis. The ENL-PMN+ve group may, therefore, be making more IL-1 than IL-6.
Differences in CRP concentration also may be due to consumption of CRP in immune complexes which are either deposited in the skin or removed from circulation during ENL, resulting in apparent lower concentrations in serum. There is a precedent for the selective removal of acute phase proteins from the peripheral circulation in amyloidosis where SAA is deposited in amyloid fibrils (6) while CRP stays in the circulation. The main role of CRP is believed to be the enhanced clearance of inappropriate materials such as microorganisms or autologous products of cell damage and death, both of which may be important in the resolution of ENL lesions in the tissues and the clearance of bacteria in the circulation. However, none of the sections examined from skin lesions of lepromatous leprosy, including ENL-PMN + ve, ENLPMN-ve and non-ENL cases, showed positive staining with anti-CRP, anti-SAA, or anti-SAP monoclonal antibodies nor with an affinity-purified polyclonal antibody to human CRP (results not shown), although a previous report demonstrated CRP both intracellularly and extracellularly in ENL lesions using a polyclonal anti-CRP antiserum (24). Furthermore, we found no difference in the concentrations of antibodies in the ENL-PMN + ve and ENL-PMN-ve groups, suggesting that the lower levels of CRP are not due to differential consumption of CRP in the two groups. Wc did find reduction in antibodies in all patients with clinical signs of ENL compared to lepromatous patients without ENL. This is consistent with earlier observations by Andecoli, et al. (1) who have reported a significant reduction in the phenolic glycolipid-I (PGL-I) antibodies in patients with ENL, as compared to the values in the same patients pre-ENL. These results strongly suggest removal of immune complexes from the peripheral circulation to other compartments. Whether CRP bound to immune complexes is selectively removed from the circulation needs confirmation, since CRP deposition in tissues has not been conclusively shown in any other inflammatory condition. Although there are basic similarities between the Arthus reaction and ENL in terms of immune complex etiology, ENL may be different in terms of the binding of CRP contributing to the inflammatory cascade (17).
In leprosy patients, the severity of an ENL reaction also is assessed by the recurrence of such episodes. Follow up of a subset of patients after the first occurrence of ENL reactions showed that the recurrence was higher in ENL-PMN + ve patients than in ENL-PMN-ve patients, although the difference did not reach statistical significance by Fisher's exact test. This observation does support the concept that the two groups of patients may have inherent differences rather than being at different stages of evolution of the ENL reaction. To address this issue we are now planning a prospective study to assess acute phase reactants in sequential serum samples taken over 7-10 days from patients presenting with ENL.
Measurement of both the acute phase proteins in ENL, therefore, provides useful clinical information; SAA as a diagnostic marker of ENL and CRP as a possible predictor of severity or disease recurrence. The lack of a more sensitive and specific "case definition" of ENL has parallels in the problems of defining, histopathologically, early leprosy (4,21), While this study was primarily an immunological study of ENL, it is evident that the clinical pathology of ENL in this population awaits full description.
Acknowledgment. This investigation received financial support from The Rockefeller Foundation and from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). We thank Dr. R. Pfau for her continuing support at the Marie Adelaide Leprosy Center in making the leprosy patients available. We also thank Dr. R. J. W. Rees for the M. leprae sonicate (batch CD 114). Extremely helpful discussions on the manuscript with Dr. A. W. Sturm are gratefully acknowledged. Finally, we thank Mrs. M. Dojki for excellent technical assistance, Mr. A. Minai for help with the statistical analysis, and Miss R. Paul for efficient secretarial assistance.
REFERENCES
1. ANDREOLI, A., BRETT, S. J., DRAPER, P., PAYNE, S. and ROOK, G. Changes in circulating antibody levels to the major phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1988)211-217.
2. CHAKRABARTY, A. K., MAIRE, M., SAHA, K. and LAMBERT, P. H. Identification of components of IC purified from human sera II; demonstration of mycobacterial antigens in immune complexes isolated from sera of lepromatous patients. Clin. Exp. Immunol. 55(1983)225-231.
3. DOUGLAS, J. T., STEVEN, L. M., FAJARDO, T., CELLONA, R. V., MADARANG, M. G., ABALOS, R. M. and STEENBERGEN, G. J. The effects of chemotherapy on antibody levels in lepromatous patients. Lepr. Rev. 59(1988)127-135.
4. FINE, P. E., JOB, C. K., MCDOUGALL, A. C, MEYERS, W. M. and PONNIGHAUS, J. M. Comparability among histopathologist in the diagnosis and classification of lesions suspected of leprosy in Malawi. Int. J. Lepr. 54(1986)614-625.
5. GILLESPIE, S. H.,Dow, C.,RAYNES,J.G., BEHRENS, R . H., CHIODINI, P. L. and MCADAM, K. P. W. J. Measurement of acute phase proteins for assessing severity of Plasmodium falciparum malaria. J. Clin. Pathol. 44(1991)228-231.
6. GLENNER, G. G. Amyloid deposits and amyloidosis; the /3-fibrilloses. N. Engl. J. Med. 302(1980)1333-1342.
7. HASAN, R., DOCKRELL, H. M., CHIANG, T. and HUSSAIN, R. Quantitative antibody ELISA for leprosy. Int. J. Lepr. 57(1989)766-776.
8. HUSSAIN, R., JAMIL, S., KIFAYET, A., FIRDAUSI, F., DOCKRELL, H. M., LUCAS, S. and HASAN, R. Quantitation of IgM antibodies to the M. leprae synthetic dissacharide can predict early bacterial multiplication in leprosy. Int. J. Lepr. 58(1990)491-502.
9. LAAL, S., BHUTANI, L. K. and NATH, I. Natural emergence of antigen reactive T cells in lepromatous leprosy patients during erythema nodosum leprosum. Infect. Immun. 50(1985)887-892.
10. MABALAY, M. C, HELWIO, E. B., TOLENTINO, J. G. and BINFORD, D. H. The histopathology and histochemistry of erythema nodosum leprosum. Int. J. Lepr. 33(1965)28-49.
11. MCADAM, K. P. W. J., ANDERS, R. F., SMITH, S. R., RUSSELL, D. A. and PRICE, M . A. Association of amyloidosis with erythema nodosum leprosum reactions and recurrent neutrophil leucocytosis in leprosy. Lancet 2(1975)572-576.
12. MELSOM, R., NAAFS, B., HARBOE, M. and CLOSS, O. Antibody activity against Mycobacterium leprae antigen 7 during the first year of DDS treatment in lepromatous (BL-LL) leprosy. Lepr. Rev. 49(1978)17-29.
13. MODLIN, R. L., MEHRA, V., JORDAN, R., BLOOM, B. R. and REA, T. H. In situ and in vitro characterization of the cellular immune response in erythema nodosum leprosum. J. Immunol. 136(1986)883-886.
14. MOSHAGE, H. J., ROELOFS, H. M. J., VAN PELT, J. F., HAZENBERG, B. P., VAN LEEUWEN, M. A., LIMBURG, P. C, AARDEN, L. A. and YAP, S. H. The effect of interleukin-1, interleukin-6, and its interrelationship on the synthesis of serum amyloid A and C reactive protein in primary cultures of adult human hepatocytes. Biochem. Biophys. Res. Commun. 155(1988)112-117.
15. MYRVANG, B. T., GODAL, T., RIDLEY, D. S., FROLAND, S. S. and SONG, Y. K. Immune responsiveness to Mycobacterium leprae and other mycobacterial antigens throughout the clinical and histopathological spectrum of leprosy. Clin. Exp. Immunol. 14(1973)541-553.
16. NAAFS, B. Reactions in leprosy. In: The Biology of the Mycobacteria, Volume 3. Clinical Aspects of Mycobacterial Disease. Ratledge, C, Stanford, J. and Grange, J. M., eds. London: Academic Press, 1989, pp. 359-403.
17. PARISH, W . E. Studies on vasculitis. VII. C-reactive protein as a substance perpetuating chronic vasculitis occurrence in lesions and concentrations in sera. Clin. Allergy 6(1976)543-550.
18. PEPYS, M . B. and BALTZ, M . L. Acute phase protein with special reference to C-reactive protein and related protein (pentaxins) and serum amyloid A protein. Adv. Immunol. 84(1983)141-211.
19. PETTIT, J. H. S. and WATERS, M. F. R. The etiology of erythema nodosum leprosum. Int. J. Lepr. 35(1967)1-10.
20. RAMANATHAN, V. D., PARKASH, O., RAMU, G., PARKER, D., CURTIS, J., SENGUPTA, V. and TURK, J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin. Immunol. Immunopathol. 32(1984)261-268.
21. RAYNES, J. G., EAGLING, S. and MCADAM, K. P. W. J. Acute phase protein synthesis in human hepatoma cells: differential regulation of serum amyloid A (SAA) and haptoglobin by interleukin 1 and interleukin 6. Clin. Exp. Immunol. 83( 1991)488-491.
22. RIDLEY, D . S. Bactériologie study of erythema nodosum leprosum. Int. J. Lepr. 28(1960)254-266.
23. RIDLEY, D. S. Reactions in leprosy. Lepr. Rev. 40(1969)77-81.
24. RIDLEY, D . S. The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr. Rev. 54(1983)95-107.
25. RIDLEY, D. S. Skin Biopsy in Leprosy. 3rd edn. Basle: Ciba-Geigy Ltd, 1990.
26. RIDLEY, D . S. and JOPLING, W . H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
27. RIDLEY, D. S., REA, T. H. and MCADAM, K. P. W. J. The histology of erythema nodosum leprosum; variant forms in New Guincans and other ethnic groups. Lepr. Rev. 52(1981)65-78.
28. STADNYK, A. W. andGAULDiE, J. The acute phase protein response during parasitic infection. Immunol. Today 12(1991) A7-A11.
29. VA N RUSWUK, M. H., RUINEN, L., DONKER, J. M., DE BLECOURT, J. J. and MANDEMA, E. Dimethyl sulfoxide in the treatment of AA amoyloidosis. Ann. NY Acad. Sci. 411(1983)67-83.
30. WEMAMBU, S. N. G., TURK, J. L., WATERS, M. F. R. and REES, R. J. W. Erythema nodosum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
1. Ph.D., M.R.C. (Path.); Department of Microbiology, Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 75800, Pakistan.
2. M.Sc; Department of Microbiology, Aga Khan University, Stadium Road, P.O. Box 3500, Karachi 75800, Pakistan.
3. M.D.; Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K.
4. Ph.D.; Department of Clinical Sciences, London School of Hygiene and Tropical Medicine, London, U.K.
5. M.B.B.S. Marie Adelaide Leprosy Center, Karachi, Pakistan.
6. M.B.B.S., M.Sc, Marie Adelaide Leprosy Center, Karachi, Pakistan.
Received for publication on 30 June 1993;
Accepted for publication in revised form on 8 November 1994.