- Volume 63 , Number 2
- Page: 295–7
Beige mice infected with Mycobacterium leprae
To the Editor:
Infection of the beige (C57/6/BG1BG1) mouse with Mycobacterium avium complex (MAC) by different routes (e.g., oral, rectal, subcutaneous, intraperitoneal, and intravenous), unlike that in BALB/c mice, results in widely disseminated disease and early mortality (6,7). Disseminated MAC infections in AIDS patients are often encountered near terminally where they commonly cause bacteremia and may contribute to the patient's demise (13). The beige mouse model of MAC infections has been used extensively by several investigators to monitor the effectiveness of antimicrobial and cytotoxic therapy whereby the level of bacilli in the liver, spleen, lungs and the blood stream, as well as survival have proved useful parameters of efficacy (2-4,9).
Recently, Gangadharam and Dhople (5) reported the utility of the beige mouse model to leprosy research. Specifically, they infected beige mice and BALB/c mice in parallel both intravenously (i.v.) and intraperitoneally (i.p.) with 1 x 107 M. leprae and in the foot pad with 6 x 103 M. leprae. They noted that while BALB/c mice infected i.v. or i.p. did not develop liver or spleen infections, beige mice infected by both routes developed infection at both sites, peaking at 4 months with M. leprae levels of 3.3- 6.2 x 105 bacilli/g of tissue which fell somewhat but was maintained at 1.3-2.1 x 105 bacilli/g at 12 months. In these studies beige mice infected in the foot pad attained levels of M. leprae peaking at 3.42 x 106 from 4 to 9 months postinfection, a level 30%-50% higher than that found in BALB/c mice infected in parallel but not at the level found in nude mice, averaging lO9foot pad (10 , 11). Because of the more luxuriant growth and dissemination found in those studies, we also infected beige and BALB/c mice in parallel utilizing both the foot pad and i.v. routes.
We infected two groups of beige mice with 5 x 103 mouse-derived and logarithmically multiplying M. leprae in either the right hind foot pad or intravenously. In parallel, a group of BALB/c mice was infected in both hind foot pads with the same M. leprae inoculum. At 8, 13, and 22 months subsequently, the number of bacilli in the right hind foot pads of one or more beige mice infected by each route was evaluated, as well as the number of M. leprae in spleens (8 and 13 months after infection), livers (8 fnonths after infection), and the contralateral left hind foot pad (8 and 13 months after infection). Also, from three beige mice infected by the foot pad route (8 and 13 months after infection) and the intravenous route (8 months after infection) various tissues were examined microscopically following both hematoxylin-and-eosin (H&E) as well as Fite-Faracco staining. These generally included the nose, tail, liver, ears, thymus, spleen, sciatic nerve, kidney, and skeletal muscle. Finally, the number of acid-fast bacilli (AFB) in four hind foot pools of BALB/c mice were enumerated microscopically 8 months after foot pad infection.
The number of M. leprae obtained by foot pad and i.v. inoculation in these studies at 8, 13, and 22 months later is presented in The Table. It is noteworthy that while M. leprae grew in BALB/c mouse foot pads to 2.21 x 105 by 5 months, the level obtained in individual right foot pads of beige mice at several time intervals was only minimally higher in one mouse and at one time interval. Furthermore, AFB from right hind foot pad-infected beige mice never disseminated to the left hind foot pad or to other organs (no granulomas or AFB seen). Of the i.v.infected beige mice there was only one instance, a spleen obtained 8 months after inoculation, wherein the presence of M. leprae was detected (2 x 105 M. leprae/foot pad or 2 x 106 M. leprae/g of tissue). In no other organ system or in the spleen at other time intervals were either granulomas or AFB found.
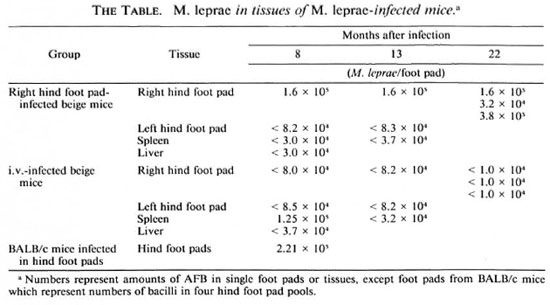
In these studies in beige mice following foot pad inoculation we found no evidence of superior growth to that of BALB/c mice and no evidence for systemic dissemination. Gangadharam, et al. (5) found essentially the same results in their foot pad-infected mice. However, the numbers of M. leprae found in beige mice in their study were slightly greater than in BALB/c mice, but not to levels obtained in nude mice (10,11) . Gangadharam, et al. (5) found that using much larger (107) i.v. and i.p. inocula than we used (5 x 103) consistent infection in the liver and spleen resulted, the intensity of which decreased with time after 5 months. In our study we found early infection in the spleen only, which resolved entirely, and no evidence of infection to the liver or elsewhere. The differences between the study done by Gangadharam, et al. (5) and our own in the level of visceral involvement following i.p. infection may well be a function of the different inoculum sizes utilized. In any event, in neither Gangadharam's nor our own study did visceral involvement of the liver and spleen approximate that found in nude mice (2 x 108/g of tissue) (12) or that in beige mice infected with MAC (108-109 bacilli/g of tissue) (1).
In beige mouse-infected foot pads local growth was not found in both studies to be substantially higher than in BALB/c mice, and not to levels in nude mice (108- 1010) or neonatally thymectomized Lewis rats (108) (8), and even i.p. or i.v. inoculation did not result in a profound, progressive, systemic infection comparable to that obtained in M. leprae- infected nude mice or MAC-infected beige mice. We, therefore, see no reason to utilize the beige mouse model in future studies of leprosy chemotherapy.
- Robert H. Gelber, M.D.
Medical Director
San Francisco Regional Hansen's Disease Program
2211 Post St., Suite 301
San Francisco, CA 94115, U.S.A.
- David M. Scollard, M.D.
Laboratory Research Branch
G. W. Long Hansen's Disease Center at Louisiana State University
P. O. Box 25072
Baton Rouge, LA 70894, U.S.A.
- Michael H. Cynamon, M.D.
Department of Infectious Diseases
Syracuse Veterans Administration Medical Center
800 Irving Avenue
Syracuse, NY 13210, U.S.A.
REFERENCES
1. BERMUDEZ, L. E. M., STEVENS, P., KOLONOSKI, P., Wu, M. and YOUNG, L. W. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant 1L-2 and tumor necrosis factor. J. Immunol. 143(1989)2996-3000.
2. BERMUDEZ, L. E., YAU-YOUNG, A. O., LIN, J. P., COGGER, J. and YOUNG, L. S. Treatment of disseminated Mycobacterium avium complex infection in beige mice with liposome-encapsulated complex infection in beige mice with liposomeencapsulated aminoglycosides. J. Infect. Dis. 161(1990)1262-1268.
3. CYNAMON, M. H., SWENSON, C. F., PALMER, G. S. and GINSBERG, R. S. Liposome-encapsulatedamikacin therapy of M. avium complex infection in beige mice. Antimicrob. Agents Chemother. 33(1989)1179-1183.
4. FERNANDEZ, P. B., HARDY, D. J., MCDANIEL, D. A., HANSON, C. W. and SWANSON, R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob. Agents Chemother. 33(1989)1531-1534.
5. GANGADHARAM, P. R. J. and DHOPLE, A. M. Utility of beige mouse in leprosy research. Indian J. Lepr. 64(1992)475-481.
6. GANGADHARAM, P. R. J., PERUMAL, V. K., FARHI, D. C. and LEBRECQUE, J. The beige mouse model for M. avium complex: optimal conditions for the host and parasite. Tubercle 70(1989)257-271.
7. GANGADHARAM, P. R. J., PERUMAL, V. K., PARIKH, K., PODAPATI, N. R., TAYLOR, R., FARHI, D. C. and ISEMAN, M. D. Susceptibility of beige mice to Mycobacterium avium complex infections by different routes of challenge. Am. Rev. Resp. Dis. 139(1989)1098-1104.
8. GELBER, R. H. The chemotherapy of lepromatous leprosy: recent developments and prospects for the future. Eur. J. Clin. Microbiol. Infect. Dis. (1994) (in press).
9. KLEMENS, S. P., CYNAMON, M. H., SWENSON, C. F. and GINSBERG, R. S. Liposome-encapsulated gentamycin therapy of M. avium complex infection in beige mice. Antimicrob. Agents Chemother. 36(1990)967-970.
10. LANCASTER, R. D., MCDOUGALL, A. C, HILSON, G. R. F. and COLSTON, M. J. Leprosy in the nude mouse. Exp. Cell Biol. 52(1984)154-157.
11. MATSUOKA, M., KAWAGUCHI, K. and KAWATSU, K. Multiplication of M. leprae in nude mice after inoculation through different routes and suitable site for growth. Int. J. Lepr. 52(1984)604.
12. MCDERMOTT-LANCASTER, R. D., ITO, T., KOHSAKA, K., GUELPA-LAURAS, C.-C. and GROSSET, J. H. Multiplication oí Mycobacterium leprae in the nude mouse and some applications of nude mice to experimental leprosy. Int. J. Lepr. 55(1987)889-895.
13. YOUNG, L. S. AIDS commentary. J. Infect. Dis. 157(1988)863-867.