- Volume 63 , Number 2
- Page: 293–395
Cell-mediated immunity in skin tuberculosis
To the Editor:
A small study was undertaken to observe the lymphoproliferative responses in the peripheral blood of patients with secondary skin tuberculosis, both before and after therapy.
Fifteen patients, 9 suffering from lupus vulgaris (LV) and 6 from scrofuloderma (SD), aged between 7 and 25 years with disease durations ranging from 3 months to 4 years, and 10 age-matched controls were studied separately for evaluation of antigeninduced lymphocyte transformation tests (LTT) to assess lymphocyte function. All of the patients and controls were clinically examined, X-rayed to rule out pulmonary tuberculosis, and blood was drawn prior to other investigations. A Mantoux test was done and read after 48 hr. The induration on the forearm measured between 16 mm and 30 mm in diameter in 13 and 10 mm in 2. Clinical diagnosis was confirmed by histopathology. The patients were then given the standard regimen (6) consisting of rifampin and isoniazid for 6-9 months supplemented during the first 2 months with pyrazinamide. The test was repeated after completion of therapy when the lesions had subsided. One patient was lost to follow up. None of the patients was taking corticosteroids during the period of study.
Soluble antigens used were purified protein derivative (PPD) of Mycobacterium tuberculosis at a concentration of 100 µg/ml and tetanus toxoid (1:10). Integral M. leprae bacilli were extracted from human lepromas as described (2), heat killed and used at an optimal concentration of 5 x lO6ml.
Peripheral blood mononuclear cells (PBMC) were separated from venous blood by the previous method (2) and suspended in RPMI-1640 with 10% fetal calf serum (FCS) at concentrations of 4-5 x lO6 100 µl of these cells were then cultured in a 96-well plate in the presence of antigens (PPD, tetanus toxoid and M. leprae) and incubated at 37ºC in a 5% humidified atmosphere. On day 6, 0.5 µCi of 3H-thymidine (BARC, Bombay, India) was added per well and incubated for a further 24 hr. Cells were then harvested on glass-fiber filter papers using a semiautomatic cell harvester (PH Environmental Div., Harris Manufacturing Co., North Billerica, Massachusetts, U.S.A.). Radioactivity was measured after adding scintillation mixture (dimethyl POPOP and diphenyloxazole PPO; Sigma Chemical Co., St. Louis, Missouri, U.S.A.) by a beta scintillation counter (Philips Scientific Analytical Div., Cambridge, U.K.). Quadruplicate cultures were set up as follows: PBMC alone, PBMC + PPD, PBMC + tetanus toxoid and PBMC + M. leprae. The mean counts per minute [cpm ± standard deviation (S.D.)] was calculated by subtracting mean cpm of cells alone from the mean cpm of cells plus antigen. Statistical analysis was performed using the Mann Whitney U test (9).
The mean cpm in unstimulated wells and in wells stimulated with antigens in the patients before and after therapy are shown in The Figure. All donors of cells had shown a significant lymphoproliferation of PBMC with PPD, TT and M. leprae antigens. The mean cpm with PBMC was 1100 ± 423.
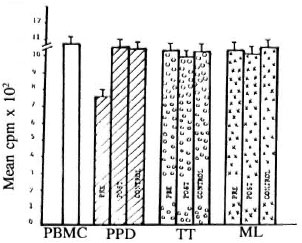
The figure. Lymphoproliferative responses of LV,SD and normal controls to PPD, tetanus toxoid andM. leprae as measured by 311-thymidine uptake andexpressed as mean counts per minute (cpm). □= PM BCresponse;  = PPD response;
= PPD response;  = tetanus toxoid re-sponse;
= tetanus toxoid re-sponse;  = M. leprae response.
= M. leprae response.
A significant depression of transformed lymphoblasts was found to PPD in patients suffering from LV and SD when compared with the control group (p < 0.005). The PPD response showed improvement after therapy and, on comparison with normal controls, it was found to be statistically significant (p < 0.01). The test did not show any significant change to M. leprae and tetanus toxoid antigens.
Unlike leprosy which shows a spectrum of skin lesions from the paucibacillary to the multibacillary types, tuberculosis of the skin usually presents as the secondary type (which is paucibacillary) with LV showing the strongest cell-mediated immunity (CMI)(8). In our study the CMI was assessed in patients suffering from LV and SD both before and after antituberculous chemotherapy. The antigens used were M. tuberculosis, tetanus toxoid and M. leprae. All patients had impaired lymphoblast transformation to PPD before therapy. This decrease was more than twofold and statistically significant. However, after therapy all of these patients regained their CMI as compared to the normal controls. The lymphoproliferative response to M. leprae and tetanus toxoid remained normal before and after treatment.
The results can be compared to tuberculoid leprosy (TT) which greatly resembles LV. Differentiating between these two conditions clinically may at times be difficult, requiring careful clinical examination since culture for M. tuberculosis is nearly always negative in LV (5). The opinion of an experienced pathologist is desirable (3). Interestingly, LTT to M. leprae antigen shows normal response in TT (1) unlike that observed before and after therapy in skin tuberculosis. Our observations are in accordance with the in vivo study which revealed no difference in the lymphocyte subsets in the peripheral blood (7) as would be expected in paucibacillary tuberculosis, but locally the dermal infiltrate revealed a pattern similar to TT with a lymphocytic mantle of CD8+ cells encircling the epithelioid granuloma which became distorted as the infiltrate increased in size (4).
These observations throw light on the clinical course of the disease as to why TT lesions often remain stable and even heal spontaneously in contrast to LV which gradually spreads in the absence of treatment.
- V. Ramesh, M.D., F.I.A.M.S.
Senior Dermatologist
Department of Dermatology and Urban Leprosy Center
Safdarjang Hospital
New Delhi 110029, India
- Uma Saxena, D.V.&D.
Chief Medical Officer (SAG)
CGHS Clinic
North Avenue
New Delhi 110001, India
- Aruna Mittal, M.Sc, Ph.D.
Deputy Director
Institute of Pathology (ICMR)
Safdarjang Hospital Campus
New Delhi 110029, India
Acknowledgment. We are grateful to the Medical Director, Hindustan Ciba-Geigy Limited, for providing a free supply of Rimactazid and PZA-CIBA. Technical assistance was provided by Ms. Madhu Badhwar.
REFERENCES
1. JOPLING, W. H. and MCDOUGALL, A. C. General principles of immunology and their application to leprosy patients. In: Handbook of Leprosy. London: Heinemann Professional, 1988, p. 72.
2. MITTAL, A. and NATH, I. Human T cell proliferative responses to particulate microbial antigens are supported by populations enriched in dendritic cells. Clin Exp. Immunol. 60(1987)611-617.
3. NIRMALA, V., CHACKO, C. J. G. and JOB, C. K. Tuberculoid leprosy and tuberculosis skin; a comparative histopathological study. Lepr. India 49(1977)65-69.
4. RAMESH, V., BENJAMIN, U. S., MISRA, R. S. and NATH, I. In situ characterisation of the infiltrate in lupus vulgaris indicates T cell proliferation. Arch. Dermatol. 126(1990)331-335.
5. RAMESH, V., MISRA, R. S. and JAIN, R. K. Secondary tuberculosis of the skin; clinical features and problems in laboratory diagnosis. Int. J. Dermatol. 28(1987)578-581.
6. RAMESH, V., MISRA, R. S., SAXENA, U. and MUKHERJEE, A. Comparative efficacy of drug regimens in skin tuberculosis. Clin. Exp. Dermatol. 16(1991)106-112.
7. SEHGAL, V. N., AHUJA, P. and SHARMA, V. K. Cellmediated immunity in cutaneous tuberculosis. Br. J. Dermatol. 118(1988)730.
8. SEHGAL, V. N., GUPTA, R., BOSE, M. and SAHA, K. Immunohistopathological spectrum in cutaneous tuberculosis. Clin. Exp. Dermatol. 18(1993)309-313.
9. SIEGEL, S. Non-Parametric Statistics for Behavioral Sciences. New York: McGraw Hill, 1956, p. 116.
Reprint requests to Dr. V. Ramesh, Sector 12/1082 RK Puram, New Delhi 110022, India.