- Volume 63 , Number 1
- Page: 48–55
Analysis of vaccines prepared f rom armadillo-derived M. leprae; results of an inter-laboratory study coordinated by the World Health Organization
ABSTRACT
Preparations of armadillo-derived Mycobacterium leprae used in vaccine trials were analyzed using a combination of morphological, chemical and immunological criteria. When compared to more recent preparations, vaccine lots prepared in 1984 and 1985 were found to contain fewer intact bacilli and lower amounts of M. leprae antigens. These differences may be characteristic of the original preparations, or alternatively, may have arisen during prolonged storage. The early vaccine lots were those used in the recently published Venezuela trial.RÉSUMÉ
Des préparations de Mycobacterium leprae provenant de tatous et utilisés pour des essais de vaccination ont été analysés sur base d'une série de critères morphologiques, chimiques et immunologiques. Comparés à des préparations plus récentes, on a trouvé que les lots vaccinaux préparés en 1984 et 1985 contenaient moins de bacilles intacts et de plus faibles quantités d'antigènes de M. leprae. Ces differences pourraient être des caractéristiques des préparations originales, ou au contraire être survenues durant leur stockage prolongé. Les lots vaccinaux anciens étaient ceux utilisés dans l'essai réalisé au Venezuela qui a fait l'objet d'une publication récente.RESUMEN
Se utilizó una combinación de criterios morfológicos, químicos, c inmunológícos, para analizar varias preparaciones de Mycobacterium leprae cultivado en armadillo que se han utilizado en distintos programas de vacunación contra la lepra. Se encontro que, comparadas con preparaciones mas recientes, los lotes de vacunas preparadas en 1984 y 1985 contuvieron menos bacilos intactos y menores cantidades de antigenos de M. leprae. Estas diferencias podrían ser características de las preparaciones origínales o, alternativamente, pudieron haberse originado durante el almacenamiento prolongada de las preparaciones vacunales. Los lotes mas antiquos fueron los usados en Venezuela durante el programa de vacunación cuyos resultados fueron recientemente publicados.A vaccine mixture containing heat-killed Mycobacterium leprae and viable BCG was tested in a randomized, double-blind, immunoprophylaxis trial carried out in Venezuela with the support of the TDR-IMMLEP unit of the World Health Organization (WHO). Over 29,000 household and nonhousehold contacts of leprosy patients were vaccinated with either the M. leprae- BCG mixture or with BCG alone, and were examined annually to detect cases of leprosy. Preliminary results of the trial (1), based on 59 cases with early lesions, showed no statistically significant difference between the incidence of leprosy in the groups vaccinated with M. leprae-BCG and with BCG alone.
Lots of the M. leprae component of the vaccine were prepared by Wellcome Laboratories, according to TDR-IMMLEP protocols, and sent to Venezuela in coded vials containing either the bacilli (6 x 108/0.4 ml) or a placebo. Vials were stored at - 20ºC, with BCG (0.1 ml) in one of two concentrations added just prior to vaccination. Subsequent to the preliminary analysis of the data from the trial, some of the vials of vaccine remaining from the trial were examined and it was noted that the turbidity of the M. leprae suspension was markedly less than that of comparable suspensions of M. leprae prepared in Venezuela. In response to this finding, a series of studies were carried out to compare batches of armadillo-derived M. leprae, coordinated by TDR-IMMLEP and involving collaboration among eight independent laboratories.
Wellcome vaccine lots. Vaccine lots were prepared by Wellcome Laboratories from leprosy bacilli purified from cobalt-irradiated infected armadillo tissues provided by the National Institute for Medical Research, Mill Hill, London, between 1976 and 1991. Sterile liver, in 50-g quantities containing not less than 109 M. leprae per gram, was processed to extract and purify M. leprae bacilli using a standard protocol [1/79]. The 5-10-ml suspensions of M. leprae obtained from each 50-g liver sample were each counted for M. leprae as acid-fast bacilli (AFB) by a single technician. Smears from each preparation were stained and assessed by Dr. R. J. W. Rees. About 700 such preparations were made and, of these, about 500 were selected as suitable for eventual pooling to be used in vaccine lots. Those deemed suitable contained 109 or more well-stained AFB/ml, with minimal clumping and minimal non-AFB debris and/or pigment. Variable numbers of acceptable preparations were pooled by Wellcome Laboratories to make up six lots for initial safety studies among Norwegian nurses, and for use in large-scale trials in Venezuela, Malawi and India (Table 1). After counting to determine the concentration of bacilli, each lot was dispensed into vials and autoclaved. The planned number of bacilli in a single-dose vial was 6 x 108, prepared at a concentration of 1.5 x 109ml. Samples from each lot were stored at - 70ºC at the National Institute for Medical Research in London, and these were made available for analysis in the studies reported here. Vials containing Lot II, which had been sent to Venezuela for the immunoprophylaxis trial in 1988 and which had been stored at - 20ºC or + 4ºC, were also included in the study, as were two batches of M. leprae purified in Venezuela from unirradiated subcutaneous nodules from infected armadillos according to the TDR-IMMLEP protocol (batch 118, prepared 5 years earlier and stored continuously at - 20ºC, and batch IP2). To carry out the analyses, samples of Lots I, II, IV and VI were prepared in coded vials in the coordinating laboratory at the London School of Hygiene and Tropical Medicine, and distributed to participating laboratories. The codes were broken only after all of the results had been submitted to the coordinating laboratory.

Counting of AFB. M. leprae suspensions for use in vaccine preparations were standardized by counting for AFB. The counts were established for each preparation prior to autoclaving. Counts were not made, routinely, after this since autoclaving causes bacterial clumping. However, for this investigation, coded samples were sent to laboratories with extensive experience in counting techniques at St. George's Hospital Medical School in London and at the Centers for Disease Control in Atlanta, Georgia, U.S.A. The results are shown in Figure 1 and summarized in Table 2. As anticipated, the estimated bacterial counts were substantially lower than those obtained before autoclaving (1.5 x 109ml), except in the case of batch 118 from Venezuela. The estimated counts for Wellcome Lots I and II were more than 10-fold lower than those established prior to bottling.
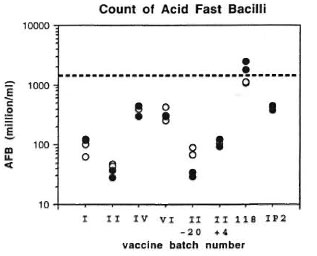
Fig. 1. Counting of AFB. Duplicate coded samples were prepared at a concentration of 1.5 x 109 ml (basedon original count estimates, ---) and recounted by lab-oratories at St. George's Hospital Medical School ( ) and at the Centers for Disease Control (
) and at the Centers for Disease Control ( ).
).
Bacterial morphology. Conventional microscopic analysis revealed the presence of nonacid-fast bacterial "ghosts" in Wellcome Lots I and II. This unusual staining characteristic had not been observed at the time of preparation of these lots. Examination of uranyl-acetate-stained preparations by electron microscopy at the Albert Einstein College of Medicine in New York City also revealed striking differences between preparations. M. leprae suspensions prepared in Venezuela contained well-preserved bacilli with very little nonbacillary debris, while samples of Wellcome Lot II contained few intact bacilli and very considerable amounts of debris (Fig. 2). Further studies with coded samples of Lots II and IV confirmed these observations for Wellcome Lot II but, in contrast, revealed the presence of morphologically intact bacilli in Lot IV.
Fig. 2. Electron microscopy analysis. M. leprae suspensions were fixed with formaldehyde-glutaraldehydemixture, and thin sections were stained for 15 min with3% uranyl acetate, and for 2 min with lead citrate. Electron micrograph in panei A shows morphologicallyintact bacilli present in a vaccine preparation fromVenezuela; panei B shows a large amount of nonbacterial debris in Wellcome Lot II.
Chemical analysis of M. leprae suspensions. The total protein content was estimated in each preparation and the sugar composition was analyzed with respect to arabinose, galactose, mannose and glucose (Table 3). The protein content was lower in Wellcome Lots I and II than in other lots- particularly Lot II when measured as the soluble protein released by sonication -and the sugar content in each of the Wellcome lots was considerably lower than that found in samples of M. leprae purified from armadillo tissues in the Department of Microbiology at Colorado State University (CSU), Fort Collins, Colorado, U.S.A. In contrast, the two Venezuelan preparations were both comparable to the CSU preparations with respect to sugar content. Figure 3 shows a gas chromatogram generated using CSU M. leprae (Fig. 3A) compared to those of Wellcome Lot I (Fig. 313) and the Venezuela preparation IP2 (Fig. 3C).
Fig. 3. Gas chromatography analysis. M. leprae preparations were hydrolyzed by treatment with trifluo-roacetic acid and sugars were then convcrted to alditolacetate derivatives which were separated by gas chromatography. Profile obtained from M. leprae prepared at CSU (A) is compared with that obtained with Wellcome Lot I (B) and with Venezuelan M. leprae batch IP2 (C).
Analysis of protein antigens. Sonicated preparations were boiled with sodium dodccyl sulfate (SDS) and subjected to Western blot analysis using monoclonal antibodies specific for M. leprae protein antigens with molecular weights 65 kDa, 28 kDa, 18 kDa and 10 kDa (Fig. 4, A-D). For each antigen strong immunoreactivity was seen for Wellcome Lots IV, V and VI and for the Venezuelan preparations. Weaker reactions were seen with Wellcome Lot I, while little or no reactivity was seen with the three independent samples of Wellcome Lot II. Immunoreactivity was observed when higher concentrations of Lot II extracts were used: sample titrations indicated, however, that the specific antigen content of Lot II was at least 10-fold lower than that of Lot VI.
Fig. 4. Western blot analysis. M. leprae preparations (1.5 x 109/m1) were sonicated and an equal volumeof SDS-PAGE sample buffer was added. After heating on a boiling water bath for 5 min, l0- µ l samples wercanalyzed by gel electrophoresis and Western blotting. Blots were developed using monoclonal antibodies specificfor the 65-kDa antigen (IIIE9, panel A); 28-kDa antigen (Bl 1H, panel B); 18-kDa antigen (L5, panel C); 10-kDa antigen (CS-01, panel D).
Guinea pig skin testing. LAt the time of preparation, each lot was tested for its ability to sensitize guinea pigs for skin-test reactivity. Guinea pigs were primed by vaccination with 4 x 109 bacilli and responses were elicited using a soluble extract of M. leprae. For each lot mean responses of >9 mm and > 12 mm were observed following challenge with 1 µ g and 6 µ g of protein, respectively. As part of the present analysis, lots were retcsted using a similar protocol. High background responses to buffer components interfered with the results (not shown), but consistent differences were apparent. Venezuelan preparations IP2 and 118 had the highest potency, followed by Wellcome Lots IV and VI and, finally, Lots I and II.
Skin-test responses in Venezuela vaccine trial. In the vaccine trial in Venezuela, 29, 113 household and other close contacts of leprosy patients were randomized to receive BCG alone or BCG + 6 x 108 M. leprae. The trial participants were resurveyed annually to detect new cases of leprosy. Also, 60 days after vaccination, 1 year after vaccination, and at annual intervals thereafter a random 10% of the participants in the trial were skin tested with tuberculin (PPD RT23) and M. leprae -soluble antigen (MLSA), the latter being prepared in several batches at the Institute of Biomcdicine in Caracas, Venezuela. Figure 5A shows the mean response (diameter of induration) to MLSA at different times after vaccination in contacts whose prevaccination response to MLSA was <10 mm. Following vaccination the skin-test response to MLSA increased in both vaccinated groups but those given the mixed vaccine showed a stronger response, and this difference between the two vaccine groups persisted for at least 5 years after vaccination.
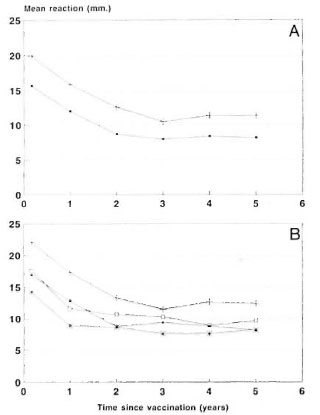
Fig. 5. Skin-test responses in Venezuela trial. A =Skin-test responses to M. leprae ' soluble antigen following vaccination with IICG or BCG + M. leprae among participants in the Venezuela trial with a pre-vaccination skin-test response <10-mm induration. ■ = BCG; + = BCG + M. leprae . B = Skin-test re-sponses to M. leprae -soluble antigen among participants receiving Lot I or Lot II of M. leprae vaccine. (Only une lot of IICG was used in the trial; graph shows skin-test responses among participants who received BCG contemporaneously with those who received thetwo lots of BCG + M. leprae. ■ = BCG (Lot I); * =BCG (Lot II); + = BCG + M. leprae (Lot I); □ = BCG + M. leprae (Lot II).
During the trial Lot II of the M. leprae vaccine was used when supplies of Lot I were exhausted; Figure 5B shows the MLSA skin-test responses separately for the two lots. The skin-test responses following vaccination with Lot II were smaller, on average, than those following Lot I, but these results are difficult to interpret because a similar difference is seen among participants who received BCG at the times Lots I and II were in use, and there was no change in the BCG used throughout the trial. The temporal differences in responses may be due, at least in part, to batch-to-batch variations in MLSA.
Conclusions. In each of the assays which we have carried out, it is clear that Wellcome vaccine Lots I and II differ significantly from later Wellcome preparations and from M. leprae batches purified in Venezuela. Lots I and II have fewer morphologically intact bacilli, have reduced sugar and total protein content, and are relatively ineffective in priming guinea pigs for skin-test responsiveness. Although comparable to the Venezuelan preparations in specific antigen content (Fig. 4), Wellcome Lots IV and VI had reduced sugar content and were less effective in the guinea pig skin-test assay. These results are fully consistent with an independent comparison of the same Venezuelan preparations with Wellcome Lot II which has been carried out by Dr. J. Convit.
There are two possible explanations for the unexpectedly low content of AFI3, carbohydrate and specific protein antigens in Wellcome Lots I and II. Either there were problems with the original M. leprae suspensions (e.g., heavily contaminated with bacterial or tissue debris) or the preparations have degraded during storage. Consistent with the latter hypothesis, Lots I and II (produced in 1984 and 1985, respectively) clearly differ from Lots IV and VI, which have been stored for only 5 and 2 years, respectively. The aberrant staining pattern (Fig. 2) was never observed at the time of original processing in any of the 700 individual M. leprae preparations (R. J. W. Recs, personal communication). Furthermore, reexamination in 1993 of samples of M. leprae preparations which had been used in Lots I and II but stored at -70ºC without autoclaving showed well-dispersed AFI3 with minimal debris and pigment, and none showed the aberrant characteristics observed in smears from vaccine Lots I and II (R. J. W. Rees, personal communication). From the quality-control criteria that were applied for original standardization-bacterial counts and guinea pig skin testing-one would have to conclude that Lots I and II had deteriorated during storage, but it is hard to account for the extensive degradation-particularly the striking absence of simple sugar molecules-observed in the studies reported here. The Venezuelan lots did not show morphological, antigenic or biochemical degradation. Available data do not allow us to exclude a third possible explanation, that human error in pooling of bacilli or in dilution could be responsible for lot variations. These results highlight the difficulties involved in standardization of a vaccine based on tissue-isolated bacteria, and focus on the need for reliable qualitycontrol tests. Western blot analysis using monoclonal antibodies represents a convenient assessment based on specific M. leprae components, while gas chromatography provides a carbohydrate "fingerprint" of each preparation which readily can be quantitated in terms of specific sugar. Both of these assays, which were not available at the time of the original vaccine production, should be used to monitor such preparations in the future.
Initial trials involving vaccination of Norwegian volunteers were used to determine the maximum acceptable dose of Wellcome Lot I, and skin-test conversion in the vaccinated population was carefully monitored in the Venezuela and in other ongoing trials. Lots I and II were effective in inducing skin-test responsiveness in the Venezuela trial, but the relationship between skin-test positivity and protective immunity remains unknown.
The evidence from this series of studies is not wholly consistent. Although there is evidence that the vaccines were immunologically potent at the time they were produced and used in the trials, the findings with respect to vaccines which had been stored for more than 5 years are disturbing. We cannot be certain whether this is an effect of storage or was a problem at the time of original production, and this clouds the interpretation that can be put on the recently published results from the Venezuela trial. Although the lots were not used concurrently, and thus the trials were not designed formally to compare them, any marked differences in protection imparted by the various lots may become apparent as cases accumulate in the Venezuela (and Malawi) trials. This may, in turn, shed valuable light on the long-standing issue of variability in protection observed by mycobacterial vaccines.
The following individuals participated in the study and in preparation of this report: London School of Hygiene and Tropical Medicine, U.K.
Dr. Saroj Young
Prof. Keith McAdam
Prof. Peter Smith
Prof. Paul Fine
National Institute for Medical Research, London, U.K.
Dr. R. J. W. Rees
St. George's Hospital Medical School, London, U.K.
Dr. Dilip Banerjee
Dr. Denise McDcrmott-Lancaster
MRC Tuberculosis and Related Infections Unit, London, U.K.
Dr. Douglas Young
Instituto de Biomedicina, Caracas, Venezuela
Dr. Jacinto Convit
Colorado State University, Fort Collins, Colorado, U.S.A.
Dr. Patrick Brcnnan
Centers for Disease Control, Atlanta, Georgia, U.S.A.
Dr. Thomas Shinnick
Laura Walker
Rosalind Van Landingham
Albert Einstein College of Medicine, New York City, U.S.A.
Dr. Barry Bloom
Statens Seruminstitut, Copenhagen, Denmark
Dr. Ase Andersen
Dr. Kaare Haslov
REFERENCE
1. CONVIT, J., SAMPSON, C., ZUNIGA, M., SMITH, P. G.,PLATA, J., SILVA, J., MOLINA, J., PINARDI, M. E., BLOOM, B. R. and Salgado, A. Immunoprophylactic trial with combined Myrobacterium leprae /BCGvaccine against leprosy: preliminary results. Lancet 339(1992)446-450.
Individuals participating in the study and in preparation of the report are shown on pages 54-55.
Reprint requests to Prof. Douglas B. Young, Ph.D., Chairman, IMMYC Steering Committee, Department of Medical Microbiology, Wright Fleming Institute, St. Mary's Hospital Medical School, Imperial College of Science, Technology and Medicine, London W2 IPG, U.K.
Received for publication on 29 June 1994.
Accepted for publication in revised form on 16 December 1994.