- Volume 63 , Number 1
- Page: 56–61
Identification and purification of armadillo (Dasypus novemcinctus) immunoglobulins: preparation of specific antisera to evaluate the immune response in these animals
ABSTRACT
In this work we describe the purification and characterization of armadillo immunoglobulins. The IgM was precipitated using low-strength ionic solution and further purified by filtration through Scphadex G-200. The IgG was obtained in pure form by precipitation of serum with ammonium sulfate and DEAE-cellulose ion exchange chromatography. The purity of these immunoglobulins was evaluated by polyacrylamide gel electrophoresis. The results showed 28-kDa light chains and 55-kDa and 70-kDa heavy chains for IgG and IgM, respectively. The rabbit antibodies against these molecules were used to prepare fluorescein (FITC) and peroxidase conjugates. The FITC conjugate was used to quantify IgM-bcaring lymphocytes. An average of 17% of peripheral blood lymphocytes were sIgM+ f rom 14 healthy animals. Additionally, in the same animals we quantified lymphocytes with the capacity to form rosettes with sheep red-blood cells; the average for this marker was 10%. Also, the production of crossreacting antibodies to BCG was evaluated in healthy and Mycobacterium leprae -inoculated animals using the peroxidase conjugates. All animals with active infection recognized BCG antigens.RÉSUMÉ
Dans ce travail, nous décrivons la purification et la caractérisation des immunoglobulincs de tatou. L'IgM a été précipitée en utilisant une solution ionique faible et purifiée par filtration à travers du Sephadex G-200. L'IgG a été obtenue sous forme pure par precipitation de serum avec du sulfate d'ammonium et chromatographic échangeuse d'ions sur cellulose DEAE. La pureté de ces immunoglobulines a été évaluée par élcctrophorèse en gel de polyacrylamide. Les résultats ont montré des chaînes légères de 28 kDa et 55 kDa et des chaînes lourdes de 70 kDa, respectivement pour les IgG et IgM. Les anticorps de lapin contre ces molécules ont été utilisés pour préparer des conjugués de lluorescéinc (FITC) et de peroxydase. Le FITC a été utilisé pour quantifier les lymphocytes porteurs d'IgM. Une moyenne de 17% des lymphocytes du sang périphérique de 14 animaux en bonne santé était positive pour les IgM. De plus, chez les mêmes animaux, nous avons quantifié la capacité des lymphocytes à former des rosettes avec des gobules rouges de mouton; la moyenne pour ce marqueur était de 10%. On a aussi évalué, avec les conjugués de peroxydase, la production d'anticorps réagissant de manière croisée au BCG chez des animaux en bonne santé et des animaux chez qui ont avait inoculé du Mycobacterium leprae. Tous les animaux avec une infection active ont reconnu les antigènes du BCG.RESUMEN
En este trabajo sc describe la purificación y caracterización de las inmunoglobulinas de armadillo. La IgM fue precipitada usando una solución de baja fuerza iónica y posteriormente purificada por filtración en Sephadex G-200. La IgG se obtuvo en forma pura por precipitación del suero con sulfato de amonio y cromatografía de intercambio iónico en DEAE celulosa. La pureza de las inmunoglobulinas se evaluó por electroforesisen gel de poliacrilamida. Los resultados mostraron cadenas ligeras de 28 kD y cadenas pesadas de 55 kD y 70 kD para IgG c IgM, respectivamente. También se prepararon anticuerpos de conejo contra estas moléculas. Los anticuerpos sc marcaron con lluorcsceína (FITC) Y peroxidasa. El conjugado con FITC sc usó para cuantilícar linfocitos con IgM membranal. En 14 animales sanos estudiados, un promedio de 17% de los linfocitos periféricos fueron sIgM4 . Adicionalmente, en los mismos animales se cuantificó la capacidad de los linfocitos para formar rosetas con eritrocitos de carnero; el promedio para este marcador fue del 10%. También se evaluó la producción de anticueropos reactivos con BCG en los animales sanos y en los inoculados con Mycobacterium leprae, usando los conjugados con peroxidasa. Todos los animales con infección activa reconocieron a los antigenos de BCG.In 1971 Kirchheimer and Storrs accomplished experimental infection of the ninebanded armadillo (Dasypus novemcinctus Linn.) with Mycobacterium leprae (7,15). The path to the study of leprosy was cleared with the availability of large quantities of bacilli to characterize useful antigens in immunodiagnosis and immunotherapy (4,5,21)- To date, however, there are only a few reports which throw light on the host-parasite relationship established between armadillos and M. leprae. This is due to the almost total lack of knowledge of the immunology of these animals (5,12,16).
In this work, we purified and characterized IgM and IgG from armadillos. Monospecific antiserums were raised in rabbits and used to evaluate immunological parameters in healthy and M. leprae -experimentally infected armadillos.
MATERIALS AND METHODS
Animals Armadillos were captured in the state of Nuevo Leon and in the high part of Michoacan state, Mexico, and were kept in captivity as described elsewhere (13). After 1-3 months of adaptation, 20 ml of blood was withdrawn from each animal by cardiac puncture. Some animals were inoculated with M. leprae from human lepromas, control animals remained untouched, and one armadillo was inoculated with bovine serum albumin (BSA) (Sigma Chemical Co., St. Louis, Missouri, U.S.A.) in complete Freund's adjuvant in order to obtain specific antibodies by affinity chromatography.
Isolation of immunoglobulins from serum
IgM. Serum (50 ml) was extensively dialyzcd against tap water. The content of the dialysis bag, primarily IgM and traces of IgG, was washed three times with chilled distilled water by ccntrifugation, and then dissolved in 5 ml of 0.1 M phosphate buffered saline (PBS) pH 5.3. IgM was further purified by filtration through Scphadcx G-200 (Pharmacia) and void volume fractions were further characterized (2).
IgG. Serum (50 ml) was precipitated three times with ammonium sulfate (33% of saturation). The precipitate was extensively dialyzed against 0.01 M phosphate buffer pH 7.5 and then fractionated by DEAE-cellulose ion exchange chromatography (Sigma). Nonabsorbed fractions were further characterized (2).
Characterization of immunoglobulins
The amount of protein was quantified by the method described by Lowry, et al. (10). The proteins were characterized by immunoelectrophoresis (2), and electrophoresis in polyacrylamide gels as was described by Laemmli (8).
Preparation of rabbit antiserum against armadillo proteins
Rabbits were inoculated, subcutancously and intramuscularly, with 3-5 mg of armadillo whole serum or purified IgM or IgG in complete Freund's adjuvant. Fifteen days later, the doses were repeated. On days 30-33 the doses were repeated again, except that those doses were inoculated without adjuvant and, finally, the rabbits were bled on day 39.
Coupling of armadillo immunoglobulins to Sepharose 4B
Sepharose 4B (Pharmacia) was cyanogenbromide-activated as described by Johnston and Thorpe (6), and 15 mg of armadillo IgM or IgG per ml of activated Sepharose was coupled. The remaining active moieties were blocked with cthanolamine. Immunoabsorbent was washed and stored in PBS-0.3% sodium azide.
Purification of rabbit antiserum
Hyperimmune sera against armadillo IgM and IgG were purified by affinity chromatography using the immunosorbents prepared as above. Each rabbit antiserum was first absorbed with an heterologous isotype immunoglobulin, thus any crossrcactivc antibody (i.e., anti-heavy or -light chain) was bound to the column. Then the unbound material was purified through a specific homologous isotype immunoglobulin.
Conjugates
The monospecific antibodies were labeled with fluorescein isothiocyanate (FITC) (Sigma) (6). Horseradish peroxidase (Sigma) conjugates were also obtained using a twostep glutaraldehydw reaction (20). Both conjugates were titrated for optimal dilution in immunofluorescence (on peripheral blood armadillo lymphocytes) (6) or ELISA using BCG sonicate as antigen (4,14).
Preparation of armadillo peripheral blood lymphocytes
The mononuclear cells were purified from peripheral blood using Histopaque 1077 (Sigma), and then stained with the FITC conjugate using standard methods (6), or evaluated for their capacity to form rosettes with sheep red blood cells (SRBC). Briefly, armadillo lymphocytes (1 x 106) were mixed with a suspension of SRBC (1 x 108). The mixture was incubated for 15 min at 37ºC, ccntrifugated at 400 x g x 3 min and then incubated overnight at 4ºC. Enumeration of rosette-forming cells was done by counting the lymphocytes with three or more SRBC attached to their surface (6).
RESULTS
Figure 1A shows the clution profile of IgM from the Sephadex G-200 column. The first peak corresponds to IgM; in peak two, we found other euglobulins. Figure 1B shows the elution profile of IgG from the DEAEcellulose column. All of the fractions from the only peak eluted contained pure IgG.
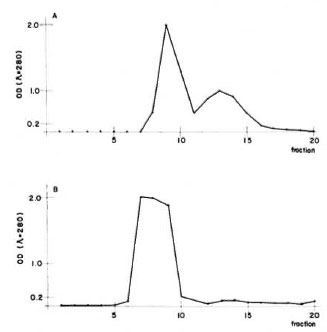
Fig 1. Purification of armadillo immunoglobulins. A = 1gM was precipitated in a low ionic strength solution from whole serum, dissolved in 0.1 M phosphatebuffered saline pH 5.3, and applied orno a Sephadex G-200 column. The first peak contained only IgM, thesecond contained other euglobulins. B = IgG was pre-cipitated from sera using a 33% final concentration ofammonium sulfate. The precipitate was dissolved in 0.01 M phosphate buffer pH 7.5 and then loaded ontoa DEAE-cellulose column. The only peak containedpure IgG.
The purity of the fractions from both columns was tested by immunoelectrophorcsis, and the results are shown in Figure 2. As can be observed, the rabbit antiserum (anti-whole scrum proteins from the armadillo) recognizes between 13-15 antigens. However, this antiserum recognizes only one protein in the first peak from the Sephadex G-200 column or in the peak from the DEAE-cellulose column as well. In order to know the purity of the fractions, they were analyzed by 10% polyacrylamidc gel electrophoresis in the presence of sodium dodecyl sulfate and 2-mercaptoethanol. The results are shown in Figure 3. IgM is separated in two fractions, one corresponding to the light chains of 28-kDa and one of 70kDa corresponding to the µ heavy chains. Similarly, IgG separates in 28-kDa light chains and 55-kDa for the heavy γ chains. In the same gel we compared the immunoglobulins of the armadillos with human gammaglobulins. Armadillo antibodies against BSA, purified by immunoaffinity, also were compared in the same gel. The results showed an equivalent molecular mobility for all the proteins.
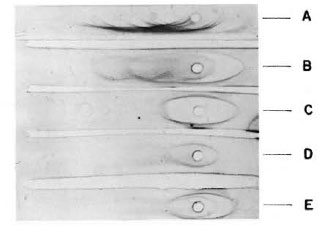
Fig. 2. Immunoelectrophoresis of serum proteinsfrom armadillo. The samples analyzed were: A = armadillo serum; B = armadillo proteins after precipitation with ammonium sulfate; C = fraction puriliedin DEAE-cellulose; D = first peak from Sephadex G-200 column; E = a mixture of samples C and D. Ali samples were developed with a rabbit antiserum to armadillo whole serum.
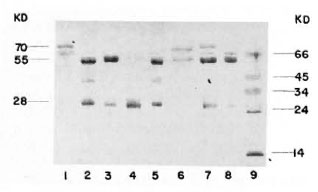
Fig. 3. Electrophoresis in polyacrylamide of thepurified immunoglobulins from armadillo. A reducing 10% SDS-PAGE was loaded with the following samples: 1 = armadillo IgM purified from the Sephadex G-200 column; 2 and 5 = human immunoglobulins (mixture of IgM and IgG); 3 = armadillo IgG purifiedfrom DEAE-cellulose column; 4 = armadillo IgG digested with papain; 6 = affinity purified anti-BSA antibodies from armadillo; 7 = a mixture of samples 1 and 3; 8 = armadillo proteins after precipitation withammonium sulfate; 9 = molecular weight markers ( α - lactalbumin 14 kDa, trypsinogen 24 kDa, pepsin 34kDa, egg albumin 45 kDa and bovine albumin 66 kDa).
The purified fractions were used to immunize rabbits, and the resulting hyperimmune sera were purified by immunoaffinity using columns of armadillo IgM and armadillo IgG coupled to Sepharose 4B. Figure 4 shows the monospecific antibodies.
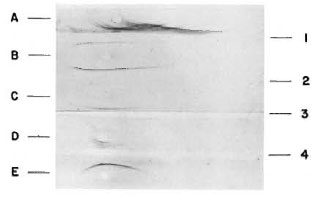
Fig. 4. Analysis of rabbit antiserum to IgM and
IgG from armadillo. The samples analyzed were: A =armadillo serum; B = armadillo proteins after precipitation with ammonium sulfate; C = armadillo IgM; D = armadillo IgM and IgG; E = armadillo IgG. Immunoelectrophoresis was developed with: 1 = rabbit antiserum to armadillo whole serum; 2 = rabbit antiserum to armadillo IgG; 3 = rabbit antiserum to armadillo IgM; 4 = rabbit antiserum to armadillo IgM and IgG.
As can be seen, crossreactivity was not detected.
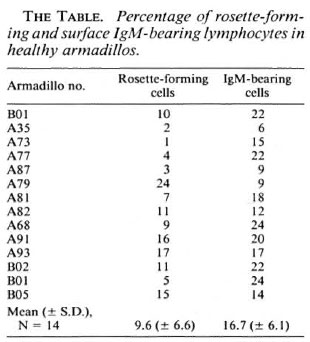
The lymphocytes bearing surface immunoglobulin M (sIgM + ) were quantified by immunofluorescence using the rabbit antibodies against armadillo IgM coupled to FITC. The rosette-forming capacity of these lymphocytes with SRBC was also evaluated. The results (The Table) show that 17% of peripheral blood lymphocytes from 14 armadillos are sIgM + , and 10% of the lymphocytes from the same armadillos form rosettes with SRBC.
Additionally, rabbit anti-armadillo IgM or IgG antibodies were peroxidase labeled in order to measure the isotype-specific, humoral immune response of healthy armadillos in comparison with those experimentally infected with M. leprae. As shown in Figure 5, animals with active infection recognized BCG crossreacting antigens with both IgM and IgG isotypes.
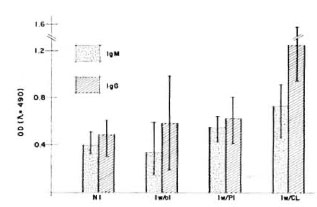
Fig. 5. Humoral immune response of M. leprae -infected versus not-inoculated armadillos. The iso-type-specific humoral immune response was evaluatedhy ELISA using a sonicated IICG as antigen. Groupsof armadillos were: Not inoculated (NI) (N = 11); inoculated but without infection (Iw/oI) (N = 6); inoculated with poor infection (Iw/PI) (N = 2); inoculated with a clearly developing infection (Iw/CI) (N = 3). Infection was evaluated by macroscopic explorationand looking for bacilli in biopsies as was described (13). Animais with poor infection did not present lepromasand the amount of bacilli was very poor at the time of the evaluation, in contrast with the clearly visible lepromas and numerous bacilli found in the other group. Ali animais were evaluated ai the same time, li months after inoculation with M. leprae .
DISCUSSION
Inoculation of BSA into armadillo induces the appearance of specific antibodies. These antibodies were purified by immunoaffinity, and were used as markers for the identification and characterization of immunoglobulins from whole serum of armadillos. Other authors have described the appearance of specific antibodies after inoculation of diverse antigens, but they have not characterized their molecules (5).
The characterization of the immunoglobulins purified from the armadillo using Immunoelectrophoresis and a rabbit antiserum against armadillo whole serum proteins demonstrated the presence of only one antigen; whereas this antiserum recognized between 13 and 15 antigens from the whole scrum of the animals in the same gel. In experiments done by Lewis and Doyle (9), five armadillo plasma fractions were detected by electrophoresis corresponding to albumin, α1, α2, β and γ globulins. Nagassi and co-workers (11) detected 20 antigens by two-dimensional immunoelectrophoresis. The differences with our results can be explained by the use of different methods and also by the quality of the antiserum used to develop the antigens in the later work.
The presence of light as well as µ and γ heavy chains was revealed by separation in polyacrylamide gel electrophoresis under reducing conditions. In this gel we were unable to identify J chains. The specific antibodies to BSA purified by immunoaffinity, and the IgM and IgG purified from Sephadex G-200 and DEAE-cellulose, when compared to human IgM and IgG, showed the same molecular weight for both heavy and light chains. These results correlate with those reported by Vadiee, et al. (18); they found that armadillo IgG have approximately the same electrophoretical mobility as human IgG. Compared with our results, they reported the same molecular weights for light chains and slightly lower molecular weights for the 7 heavy chains. The difference in the molecular weights of the 7 heavy chains is not significant (only 4-kDa difference) and can be easily explained by the experimental error of the method. We found that the rabbit antiserums to IgM had crossreactivity with IgG and vice versa (data not shown). Thus, in order to work with monospecific antibodies, immunoabsorption with their heterologous and homologous immunoglobulins coupled to Sepharose 4B was performed. Using this method we prepared highly specific reagents to analyze the cellular and humoral immune responses in the armadillo.
The identification of armadillo lymphocyte markers is important for the description and quantification of lymphoid populations in these animals. This is pertinent information for scanning the possible alterations in these cell populations during infection. In this work we identified and quantified lymphocytes bearing surface IgM (a characteristic B-lymphocyte marker), and rosette-forming lymphocytes with SRBC (a human T-lymphocyte marker), as well. Baliña(1) reported 27.13% for sIgM+ populations in D. hybridus, while we found only 16.7% in D. novemcinctus. The different values can be explained since we dealt with a different species and because we quantified only the IgM-bcaring lymphocytes.
For a rosette-forming lymphocyte population, Baliña (1) reported 7.27% in D. hybridus and Escobar, et al. (3) found values less than 49% in D. novemcinctus; our data show values of 9.6% for this cell type. These low values are very far from those reported for man, thus "a priori" it may be speculated that such a method is not good enough to identify armadillo T lymphocytes.
In this work, the humoral response of healthy and experimentally M. leprae- infected armadillos against a crossreacting antigen (sonicated BCG) was measured. Determination of isotype-specific antibodies against BCG epitopes demonstrated a rising response of specific IgM and IgG in the armadillos when the infection has been established. In close correlation with other reports (17,19), this work shows that these animals are capable of recognizing and reacting against crossreactive antigens on mycobacterial species.
We conclude that in armadillos it is possible to evaluate the humoral immune response as the M. leprae infection progresses. On the other hand, more work is needed to identify the lymphoid populations of D. novemcinctus.
Acknowedgment. This work was supported by grants from the Consejo Nacional de Ciencia y Tecnología, México, D.F., México, and Dirección de Estudios de postgrado e Investigación, I.P.N., México. The authors hold fellowships from COFAA, BDA and/or SNI, México.
REFERENCES
1. BALIÑA, L. M., CARDAMA, J. E., GATTI, J. C, VALDEZ, R. P. and BlANCHI, O. Research on armadillos in Argentina. In the armadillo as an experimental model in biomedical research. Pan American Health Organization, Scientific Publication 366(1978)103-106.
2. CAMPBELL, D. H., GARVEN, J. S., CREMER, N. E. and SUSSDORF, D. H., eds. Methods in Immunology. London: W. A. Benjamin Inc., 1970, pp. 193-201.
3. ESCOBAR-GUTIERREZ, A. and AMEZCUA, M. E. El armadillo: un nuevo animal de experimentación para el estudio de las zoonosis. Ciencia Veterinaria 3(1981)200-224.
4. ESTRADA-GARCÍA, L, QUESADA-PASCUAL, F., SANTOS-ARGUMEDO, L., FLORES-ROMO, L., ESTRADA-PARRA, S. and BUCHANAN, T. M. The early serodiagnosis of leprosy. I. The use ofcounter-immunoclectrophoresis and enzyme-linked immunosorbent assay. Rev. Latino-am. Microbiol. 26(1984)267-272.
5. HARBOE, M. Significance on antibody studies in leprosy and experimental models of the disease. Int. J. Lepr. 50(1982)342-350.
6. JOHNSTONE, A. and THORPE, R. Immunochemistry in Practice. Oxford: Blackwell Scientific Publication, 1982, pp. 150-162; 202-209; 256-259.
7. KIRCHHEIMER. W. F. and STORRS, E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn) as a model for study of leprosy. Int. J. Lepr. 39(1971)693-702.
8. LAEMMLI, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(1970)680-685.
9. LEWIS, J. H. and DOYLE, A. P. Coagulation proteins and cellular studies on armadillo blood. Comp. BIOCHEM. Physiol. 12 (1964) 61-66.
10. LOWRY, H. O., ROSEBROUGH, N. I., FARR, A. L. and RANDALL, R. J. Protein measurement with the folin phenol reagent. J. Biol. Chcm. 193(1951)265-275.
11. NEGASSI, K., CLOSS, O. and HARBOE, M. Crossreaction between scrum proteins and water soluble liver tissue antigens in the nine-banded armadillo (Dasypus novemcinctus Linn) and man. Clin. Exp. Immunol. 38(1979)135-147.
12. PURTILO, G. P. and STORRS, E. E. Impact of cool temperatures on transformation of human and armadillo lymphocytes (Dasypus novemcinctus Linn) as related to leprosy. Nature 248(1974)450-452.
13. QUESADA-PASCUAL, R., ROJAS-ESPINOSA, O., SANTOS-ARGUMEDO, L. and ESTRADA-PARRA, S. A Mexican armadillo (Dasypus novemcinctus) colony for leprosy research. Int. J. Lepr. 55(1987)716-718.
14. SANTOS-ARGUMEDO, L., HERNÁNDEZ-LÓPEZ, J., Quesada-Pascual, F. and ESTRADA-PARRA, S. Estandarización del inmunoanálisis enzimático. infectologia 6(1986)18-23.
15. STORRS, E. E. The nine-banded armadillo: new model for biomedical research. Int. J. Lepr. 39(1971)703-713.
16. ULRICH, M., CONVIT, J. and RAPETTI, M. Immunological characteristics of the armadillo Dasypus sabanicola. Clin. Exp. Immunol. 25(1976)170-176.
17. VADIEE, A. R., HARRIS, E. and SHANNON, E. J. The evolution of antibody response in armadillos inoculated with Mycobacterium leprae. Lepr. Rev. 61(1990)15-226.
18. VADIEE, A. R., SHANNON, E. J., GILLIS, T. P. and HASTINGS, R. C. Partial characterization of antigens from M. leprae evoking IgG and IgM antibodies in armadillos. Int. J. Lepr. 56(1988)274-281.
19. VADIEE, A. R., SHANNON, E. J., GILLIS, T. P., MSHANA, R. N. and HASTINGS, R. C. Armadillo IgG and IgM antibody responses to phenolic glycolipid-I during experimental infection with M. leprae. Int. J. Lepr. 56(1988)422-427.
20. VOLLER, A., BIDWELL, D. E. and BARTLETT, A. The enzyme-linked immunosorbent assay (ELISA). London: Dynatcch Laboratories Inc.
21. YOUNG, D. R. and BUCHANAN, T. M. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science 221(1983)1057-1059.
1. Ph.D., Department of Cell Biology, Centro de Investigación y Estudios Avanzados, I.P.N., Apartado Postal 14-740, Mexico, D.F. 07000, Mexico.
2. M.Sc, Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpio y Plan de Ayala, Mexico, D.F. 11340, Mexico.
3. M.Sc, Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpio y Plan de Ayala, Mexico, D.F. 11340, Mexico.
4. Ph.D., Department of Immunology, Escuela Nacional de Ciencias Biológicas, I.P.N., Prol. Carpio y Plan de Ayala, Mexico, D.F. 11340, Mexico.
Received for publication on 21 January 1994.
Accepted for publication in revised form on 11 October 1994.