- Volume 63 , Number 1
- Page: 1–7
Are 18 Doses of WHO/MDT sufficient for multibacillary leprosy; results of a trial in Malawi
ABSTRACT
A trial comparing 18 monthly and 30 monthly doses of the World Health Organization-recommended multidrug therapy (WHO/MDT) in 305 multibacillary leprosy patients in Malawi is described. Patients were randomly allocated to one of the two regimens at the time of taking the 18th supervised dose of WHO/MDT. The mean follow-up period was 3 years (maximum 6 years). No relapse was observed in either group. The cumulative probabilities of remaining slit-skin smear positive were significantly higher among patients receiving only the 18 monthly doses of WHO/MDT, but reached zero at month 60 of follow up. The percentage of patients who developed new disabilities during the trial period was similar in both groups. However, the overall percentage of patients who developed new disabilities (50/305, 16.4%) remains disturbingly high. On the whole, the results of the trial argue in favor of 18 monthly doses of WHO/MDT taken within 24 months as being sufficient for the treatment of multibacillary leprosy.RÉSUMÉ
On décrit un essai comparatif de 18 et 30 doses mensuelles de la polychimiothérapie aux doses recommandées par l'Organisation Mondiale de la Santé (PCTV OMS). Les patients ont été répartis au hasard pour recevoir l'un ou l'autre des deux régimes thérapeutiques au moment de recevoir leur dixscpticmc dose de PCT/OMS. La période moyenne de suivi a été de 3 ans (maximum 6 ans). Aucune rechute n'a été observée dans aucun des deux groupes. Les probabilités cumulées de rester positif aux frottis cutanés étaient sigificativement plus élevées pour les patients qui ont reçu seulement 18 doses mensuelles de PCT/OMS, mais ont atteint zéro au soixantième mois du suivi. Le pourcentage de patients qui ont développé de nouvelles incapacités durant la période d'essai était semblable dans les deux groupes. Cependant, le pourcentage total des patients qui ont développé de nouvelles incapacités (50/305, 16.4%) reste élevé de manière troublante. Dans l'ensemble, les résultats de l'essai plaident en faveur du fait que les 18 doses mensuelles de PCT/OMS prises dans un intervalle de temps de 24 mois seraient suffisantes pour le traitement de la lèpre multibacillaire.RESUMEN
Se hizo un estudio en 305 pacientes con lepra de Malawi, comparando la efectividad de 18 y 30 dosis mensuales de la poliquimioterapia (PQT) recomendada por la Organización Mundial de la Salud (OMS). Los pacientes se repartieron al azar en cada uno de los dos esquemas de tratamiento, cuando ya habian recibido 18 dosis de la PQT/OMS. El periodo promedio de seguimiento fue de 3 años (máximo de 6 años). En ningún grupo se observaron recaídas. Las probabilidades acumulativas de permanecer positivos en las extensiones de linfa cutánea fueron significativamente mayores entre los pacientes que recibieron sólo las 18 dosis de la PQT/OMS, pero fueron de cero hacia el mes 60 de sequimiento. El porcentaje de pacientes que desarrollaron nuevas alteraciones incapacitantes durante el periodo que duró el ensayo fue similar en ambos grupos. Sin embargo, el porcentaje global de pacientes que desarrollaron nuevas alteraciones incapacitantes (50/305, 16.4%) permaneció preocupantemente alto. De manera general, los resultados del estudio cstñ en favor de las 18 dosis mensuales de la PQT/ OMS, tomadas dentro de un periodo de 24 meses, como suficientes para el tratamiento de la lepra multibacilar.A study was initiated in 1987 among multibacillary (MB) patients in Malawi within the framework of the LEPRA Control and Evaluation Projects and the Shire Valley Leprosy Control Project to investigate whether treatment of MB patients can be shortened without increasing the number (percentage) of unfavorable outcomes. Until the beginning of the study, MB patients in Malawi were treated with 100 mg of dapsone and 50 mg of clofazimine daily as well as 600 mg of rifampin and 300 mg of clofazimine supervised monthly, as recommended since 1982 by the World Health Organization (WHO) (13). This multidrug treatment (MDT) was given for at least 24 months and continued thereafter until two consecutive slit-skin smears showed bacterial indexes (Bis) of 0 in line with WHO recommendations (13). Several countries adopted a policy of only 24 months' treatment even if one or more slit-skin smears were still positive at that time.
In the absence of any definitive long-term study in which MB patients received 24 monthly doses of MDT, we decided to compare different durations of the above described MDT: 18 versus 30 monthly doses (i.e., 6 months shorter versus 6 months longer than the recommended minimum of 24 months).
If long-term outcomes after 18 monthly doses of WHO/MDT are at least as good as long-term outcomes after 30 monthly doses, then such a result would open the way a) to introduce 18-month treatment regimens for MB leprosy and b) to study even shorter WHO/MDT durations for MB leprosy patients. Shorter treatment regimens allow for easier patient compliance and perhaps even more importantly, makes it easier to implement and sustain a national program.
Long-term results have to be measured in terms of relapse rates and incidence rates of disabilities during and after MDT; the latter being the more important since disabilities are the main problem for patients and make leprosy a public health problem. Relapse rates are probably of lesser importance as long as relapses can be treated successfully.
METHODS
For the purpose of this study a MB leprosy patient was defined as a) any untreated leprosy patient with a BI of 2 or higher in a slit-skin smear from any site or b) any untreated patient in whom a skin or nerve biopsy yielded evidence of MB leprosy or c) any newly registered relapse with evidence of MB leprosy.
The BI was measured according to the (logarithmic) Ridley scale (9). Slit-skin smears were taken from both earlobes and from at least two skin lesions at registration (the beginning of treatment).
We only recruited patients into the study who were born after 1918, lived in an area accessible at least by motorcycle, and after they had received 18 supervised monthly doses of 600 mg rifampin plus 300 mg clofazimine within 24 months of registration. At this point in time patients were randomly allocated to either stop antileprosy treatment or to take a further 12 monthly doses of MDT to make a total of 30 doses (which had to be taken by the patient within 40 months of registration). The method adopted was that the leprosy control assistants (LCAs) looking after the patients opened a sealed envelope with the instruction for either 18 or 30 doses at the time of giving the 18th monthly dose of WHO/MDT.-The procedure was closely supervised by senior stafT. Overt type 1 reaction and recent deficit in nerve function were treated with steroids as described previously (2). Severe or recurrent type 2 reactions were treated with 300 mg clofazimine daily tapered down to zero over 18 months. Dapsone and rifampin were discontinued in these patients according to their treatment group.
Table 1 shows the frequency distribution of the highest Bis in slit-skin smears at the time of registration by treatment group. The mean BI in the 18-dose group was 3.92; the mean BI in the 30-dose group was 3.89. The highest BI was < 2 in 25 of the 305 patients recruited into the study. In these patients there was histopathological evidence of MB leprosy (see b above). Of the 305 patients, 259 (84.9%) were new untreated cases, 133/157 in the 18-dose group and 126/148 in the 30-dose group.
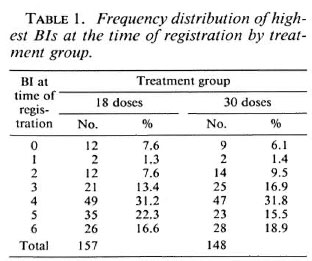
At the time of randomization 75 (47.8%) patients allocated to stop treatment had a BI of 0; the corresponding figure for patients allocated to a further 12 doses of WHO/ MDT was 68 (45.9%).
The age distribution of patients at the time of registration by treatment group is shown in Table 2. Nearly half the patients in each group were 30 to 44 years old. In each treatment group a third of the patients were female.
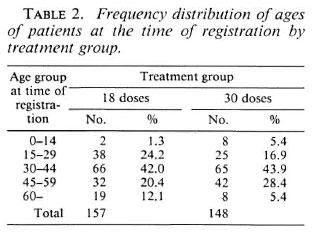
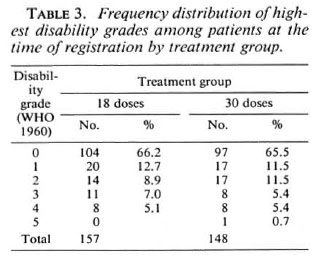
For the grading of disabilities, wc used the 1960 WHO classification (12); grade 1 = anesthesia of hands and/or feet, grade 5 = extensive loss of fingers and/or loss of more than one third of a foot and/or blindness due to leprosy. The term marked disability as used for the outcome with respect to disabilities implies at least one of the following: lagophthalmos, paralysis or loss of a finger, a plantar ulcer or a foot drop. At the time of registration 124 (79.0%) patients in the 18-dose group and 114 (77.0%) patients in the 30-dose group had no disabilities although some of them had anesthesia (Table 3).
After randomization patients were reviewed by LCAs every 6 months, usually at their homes. At each review a full clinical examination was carried out and slit-skin smears were taken from the same sites as at registration. Smears were examined by laboratory assistants without knowing previous results. The following definition of a relapse was decided upon before the start of the trial: a) MB relapse: if the highest BI of any site shows an increase > 2+ compared with the highest BI of any site in the previous smears. This new result might be from the smears of an old lesion or from the smear of a new lesion. Such an increase in BI should be accompanied by an increase in the proportion of fragments + solids. The percentage of solids + fragments in the actual smear minus the percentage of solids + fragments in the previous smear should be > 10%. b) PB relapse: definite clinical evidence of PB leprosy found by one of the investigators and/or definite histopathological evidence of PB leprosy in a new lesion.
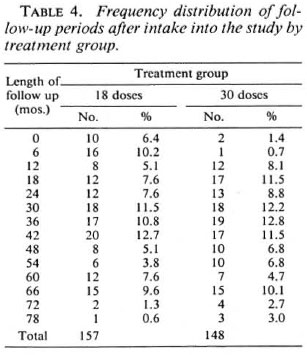
The envisaged follow-up period was 5 years after randomization. The actual distribution of follow-up periods is shown in Table 4 and Figure 1. The mean follow-up periods were 33.9 months and 38.2 months for patients allocated to 18 and 30 doses of WHO/MDT respectively.
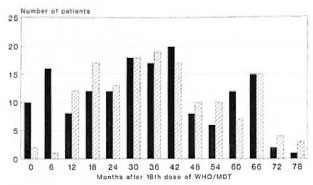
Fig. 1. Frequency distribution of follow-up periodsafter 18 doses of WHO/MDT by treatment group.  = 18-dose group;
= 18-dose group;  = 30-dose group.
= 30-dose group.
The human immunodeficiency virus (HIV) status of patients was ascertained only at registration in the area covered by the LEPRA Evaluation Project but results were not used in this analysis (8).
Data were entered on computers and tabulated using EPI INFO software.
RESULTS
No relapse was observed in either treatment group.
Table 5 and Figure 2 show conditional and cumulative probabilities of patients remaining slit-skin smear positive after 18 doses of WHO/MDT by treatment group (1,3), At the time of randomization 82 of the 157 patients allocated to 18 doses (M18) and 80 of the 148 patients allocated to 30 doses (M30) still had a highest BI of > 1. The BI of all patients, as far as the patients remained available for review, fell to 0 by 60 months. From month 6 onward cumulative probabilities of remaining slit-skin smear positive were constantly higher for the M18 group than for the M30 group. The difference is statistically marginally significant (log rank test, x2 = 4.23, p < 0.05).
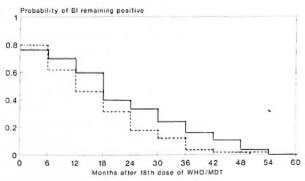
Fig. 2. Cumulative probabilities of remaining slit-skin smear positive after 18 doses of WHO/MDT by treatment group. _____ = I8-dose group; ---- = 30-dose group.
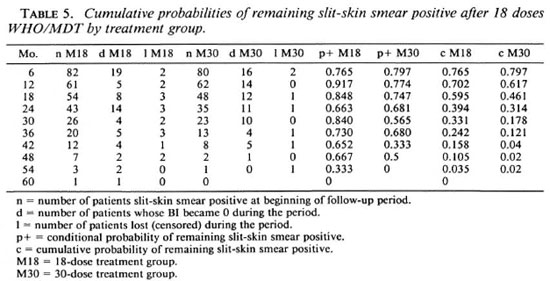
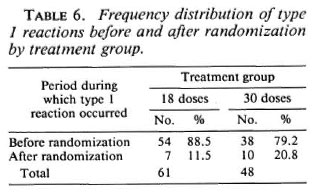
Among the patients allocated to 18 doses, 61 (38.9%) were found cither with a recent deficit in nerve function or developed at least one overt type 1 reaction; among those allocated to 30 doses the number was 48 (32.4%). Table 6 shows that the vast majority of type 1 reactions occurred before randomization and that considerably more were observed in the M18 than in the M30 group.
The results of review examinations with respect to disabilities are shown in Table 7. Of the 157 patients allocated to 18 doses of WHO/MDT, 86 (54.8%) never had any disability; of the 148 patients allocated to 30 doses, the number was 87 (58.8%). Altogether 50 patients developed minor or marked new disabilities, 30 (19.1%) in the M18 group, 20 (13.5%) in the M30 group. The difference is statistically not significant (Mantel-HanBcl, p = 0.19). Another 18 and 12 patients, respectively, experienced a worsening of their disabilities in the two treatment groups. If these are combined with those patients who developed new disabilities, the difference between the two treatment groups approaches statistical significance (p = 0.076).
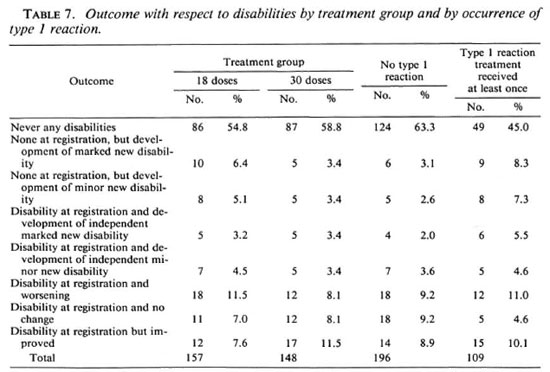
The relationship between episodes leading to treatment with steroids (either because of recent nerve damage or because of overt type 1 reactions) and the outcome with respect to disabilities is also shown in Table 7. There is a highly significant relationship between the two (p = 0.006). For example, 17 (9 + 8) of 109 patients (15.6%) with a type 1 reaction developed new disabilities, while only 11 (6 + 5) of 196 patients (5.7%) who did not have a reaction suffered new disabilities. This underlines the fact that type 1 reactions are an important risk factor for the development of disabilities and also that the present steroid treatment, even if instituted quickly, is unsatisfactory.
Severe type 2 reactions occurred in only 9 patients; of these 9 patients 5 were in the M18 group, 4 in the M30 group.
DISCUSSION
There have been a number of trials during the last couple of years trying out new and shorter treatment regimens for MB leprosy (4-7, 10) Our trial is different insofar as we only varied the period of administering a generally recommended and widely accepted treatment regimen (WHO/MDT). This seemed of considerable interest to us in particular since the duration of the recommended regimen was not based on the results of a trial. If 30 doses of WHO/MDT had resulted in a better outcome than 18 doses, the wisdom of giving MDT for only 2 years would have been thrown in doubt.
For practical purposes our trial was not double-blind. Mostly we were concerned that placebo and actual drug treatment (WHO/MDT) might get confused by our field staff. In addition, we were not sure that it would be ethical to ask patients to walk every month to a treatment point in order to receive a placebo. One effect of this procedure was, unfortunately, that a number of patients in the M18 group were lost early on to follow up after randomization. This explains why the average duration of follow up is shorter for this group (Table 4).
Allocation was randomized as described, and we have shown that randomization was largely successful: patients in the two treatment groups were comparable with regard to age, sex, Bis at registration, and the frequency distribution of disabilities at registration. Only with regard to the occurrence of type 1 reactions did our groups differ in that more type 1 reactions were observed before randomization in the M18 group. Given the relationship between type 1 reaction and the development of new disabilities, this imbalance needs to be discussed further.
Although a number of patients in the trial must have been HIV positive, no relapse was observed in either treatment group. This in contrast to the experience in Mali (5) where 2.9% of a small number of patients developed a relapse during years 3 and 4 of follow up after completion of 24 doses of WHO/ MDT. Our results indicate that 18 doses of WHO/MDT may be sufficient concerning relapses. They also show the superiority of WHO/MDT over dapsonc monotherapy with regard to relapses (11). On the other hand it still can be argued that a late relapse rate might be higher among patients treated with 18 doses of WHO/MDT than among those treated with 30 doses. This only time will tell.
We have also shown that even without further antileprosy treatment Bis still positive at the time of randomization became 0 in due course. We did, however, observe an interesting difference in the cumulative probabilities of remaining slit-skin smear positive in that patients in the M18 group had a significantly higher cumulative probability of remaining slit-skin smear positive than patients in the M30 group. This argues against reducing the number of doses of WHO/MDT below 18 without a trial.
We further observed that more patients in the M18 group developed disabilities than did patients in the M30 group. While this is most disturbing at first sight, the difference has to be seen in light of the already mentioned fact that more type 1 reactions occurred in patients allocated to the M18 group. However, because the vast majority occurred before randomization (Table 6) this imbalance can only be due to chance. Thus, even the outcome with respect to disabilities is essentially the same in the two groups. This, again, argues in favor of 18 doses of WHO/MDT being sufficient. On the other hand, the overall outcome of antileprosy treatment of MB patients leaves a lot to be desired. As can be seen from Table 7, the percentage of patients who suffered worsening of disabilities (30/305; 9.8%) or new disabilities (50/305; 16.4%) continues to be disturbingly high. The ultimate treatment for MB leprosy has surely not been found yet, and we propose that consideration should be given to the feasibility of a trial in which antiinflammatory drugs are included routinely in the antileprosy treatment for the first 12 to 18 months after registration, during which time most type 1 reactions occur.
Finally, we would like to mention the small number of type 2 reactions observed by us. Presumably this is due to the clofazimine component of WHO/MDT.
Acknowledgment. Basic funding for the LEPRA Evaluation Project, the LEPRA Control Project, and the Shire Valley Leprosy Control Project is provided by the British Leprosy Relief Association (LEPRA) and other ILEP members. We wish to thank Dr. T. Macrery, Mr. L. Chinyama, Mr. P. Katumbi, and all the LCAs looking after the patients. Dr. S. Lucas read the biopsies. Our thanks also go to the Ministry of Health of the Republic of Malawi for permission to publish this paper.
REFERENCES
1. ARMITAGE, P. and BERRY, G. Statistical Methods in Medical Research. 2nd edn. Oxford: Blackwell Scientific Publications, 1987.
2. BOERRIGTER, G., PÖNNIGHAUS, J. M. and FINE, P. E. M. Preliminary appraisal of a WHO-recommended multiple drug regimen in paucibacillary leprosy patients in Malawi. Int. J. Lepr. 56(1988)408-417.
3. CLAYTON, D. and HILLS, M. Statistical Models in Epidemiology. Oxford: Oxford Science Publication, 1993.
4. GROSSET, J. H., Ji, B., GUELPA-LAURAS, C-C, PERANI, E. G. and N'DELI, L. N. Clinical trial of pefloxacin and ofloxacin in the treatment of lepromatousleprosy. Int. J. Lepr. 58(1990)281-295.
5. MARCHOUX CHEMOTHERAPY STUDY GROUP (prepared by Jamet, P. and Ji, B). Relapses in multibacillary leprosy patients after stopping treatment with rifampin-containing combined regimens. Int. J. Lepr. 60(1992)525-535.
6. PATTYN, S. R., BOURLAND, J. and KAZEZE. Ambulatory treatment of multibacillary leprosy with a regimen of 8 months duration. Lepr. Rev. 63(1992)36-40.
7. PATTYN, S. R., GROENEN, G., JANSSENS, L., KUYKENS, L. and MPUTU, L. B. Treatment of multibacillary leprosy with a regimen of 13 weeks duration. Lepr. Rev. 63(1992)41-46.
8. PONNIGHAUS, J. M., MWANJASI, L. J., FINE, P. E. M., SHAW, M., TURNER, A. C, OXBORROW, S. M., LUCAS, S. B., JENKINS, P. A., STERNE, J. A. C. and BLISS, L. Is HIV infection a risk factor for leprosy? Int. J. Lepr. 59(1991)221-228.
9. RIDLEY, D. S. Therapeutic trials in leprosy using serial biopsies. Lepr. Rev. 29(1958)45-52.
10. THOMAS, A., BALAKRISHNAN, A., NAGARAJAN, M., PRABHAKAR, R., TRIPATHY, S. P., CHRISTIAN, M. and SOMASUNDARAM, P. R. Controlled clinical trial of two multidrug regimens with and without rifampin in highly bacillifcrous BL/LL South Indian patients: a five-year report. Int. J. Lepr. 58(1990)273-281.
11. WATERS, M. F. R., REES, R. J. W., LAING, A. B. G., KHOO KAH FAH, MEADE, T. W., PARIKSHAK, N. and NORTH, W. R. S. The rate of relapse in lepromatous leprosy following completion of twenty years of supervised sulphone therapy. Lepr. Rev. 57(1986)101-109.
12. WHO EXPERT COMMITTEE ON LEPROSY. Second report. Geneva: World Health Organization, 1990. Tech. Rep. Ser. 189.
13. WORLD HEALTH ORGANIZATION. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
1. Dr. Med., D. T. P. H., Universitats-Hautklinik, 66424 Homburg, Saar, Germany.
2. M.D., P. O. Box 90, Bvumbwe, Malawi.
Received for publication on 6 July 1994.
Accepted for publication on 13 September 1994.