- Volume 63 , Number 1
- Page: 8–17
Minocycline in lepromatous leprosy
ABSTRACT
Twelve patients were treated with three dose levels of minocycline for 30 days, primarily to detect the dose-related effects on Mycobacterium leprae viability, followed by another 5 months of daily minocycline for overall efficacy and persistence of clinical and antibacterial effects. Subsequently, the patients were given standard WHO/MDT chemotherapy for multibacillary leprosy. Clinical improvement was recognizable during the first month, occurring much earlier among those on minocycline 200 mg daily than those who received minocycline 100 mg daily. A similar change also was observed in one patient 11 days after three daily doses of 100 mg of minocycline. At the end of 6 months, all patients were clinically improved with a slight reduction in the average bacterial index (BI) and logarithmic index of bacilli in biopsy (LIB). The effects of minocycline on viability by mouse foot pad inoculation and palmitic acid oxidation assays were noted beginning at 10 to 14 days of daily dosing and becoming more definite after 30 days of treatment. Both tests correlated fairly well. Doses of 200 mg daily did not appear to be more efficient than minocycline 100 daily. Phenolic glycolipid-I (PGL-I) antigen determinations done on some patients during the first month remained positive and did not correlate with changes in viability results. At the end of 6 months, after 5 months of 100 mg of minocycline monotherapy, no viable organisms could be demonstrated by mouse foot pad inoculation and palmitic acid oxidation assays; assays for PGL-I antigen were all negative. No lepra reactions were observed during the 6 months of therapy. Tolerable side effects, dizziness and abdominal discomfort were noted only during the first week of treatment in 2 of 12 patients. A generalized light-brown pigmentation was observed, which was more intense and blue-gray over the sites of subsided localized lesions. The results of this study further confirm the early effects of minocycline on clinical lesions and the viability of M. leprae with antibacterial and clinical effects becoming definitely more demonstrable after 6 months of treatment. Thus, minocycline is a valuable drug in the treatment of leprosy, and studies to determine its efficacy in combination with other antileprosy drugs, dosage levels and pulsed dosaging, as well as the effects of lepra reaction, should be pursued.RÉSUMÉ
Douze patients ont été traités avec trois dosages de minocycline pendant 30 jours, tout d'abord pour détecter les effets liés au dosage sur la viabilité du Mycobacterium leprae; cette période a été suivie de 5 mois de minocycline quotidienne pour étudier l'efficacité globale et la persistance d'effets cliniques et antibactériens. Par la suite, les patients ont reçu une chimiothérapie PCT/OMS standard pour la lèpre multibacillairc. Une amélioration clinique a été reconnue durant le premier mois, survenant beaucoup plus tôt parmi ceux qui recevaient 200 mg de minocycline par jour que chez ceux qui recevaient 100 mg de minocycline par jour. Un changement semblable était également observé chez un patient 11 jours après trois doses quotidiennes de 100 mg de minocycline. A la fin des 6 mois, tous les patients étaient améliorés cliniquement, avec une légère réduction de l'indice bactérien (IB) moyen et de l'indice logarithmique de bacilles (ILB) dans la biopsie. On a noté que les effets de la minocycline sur la viabilité mesurée par inoculation dans le coussinet plantaire de souris et des tests basés sur l'oxydation de l'acide palmitique commençaient du lOème au 14ème jours du traitement quotidien, et devenaient plus nets après 30 jours de traitement. Il y avait une assez bonne correlation entre ces deux tests. Des doses de 200 mg par jour n'apparaissaient pas plus efficaces que 100 mg de minocycline par jour. Les déterminations d'antigène du glycolipide phénolique I (PGL-I) réalisée chez certains patients durant le premier mois sont restées positives et ne montraient pas de correlation avec des changements dans les résultats de viabilité. A la fin des 6 mois, après 5 mois de monothérapie par 100 mg de minocycline, aucun organisme viable ne pouvait être mis en évidence par inoculation dans le coussinet plantaire de la souris et des tests d'oxydation de l'acide palmitique; les tests pour l'antigène PGL-I étaient tous négatifs. Aucune réaction lépreuse n'a été observée durant les 6 mois de traitement. Des efTets secondaires supportables, des vertiges et une gène abdominale n'ont été notés que durant la première semaine de traitement chez 2 des 12 patients. On a observé une pigmentation généralisée brun-clair, qui était plus intense et bleu-gris aux endroits des anciennes lésions localisées. Les résultats de cette étude confirment les effets précoces de la minocycline sur les lésions cliniques et la viabilité de M. leprae, avec des effets anti-bactériens et cliniques devenant nettement plus démontrables après 6 mois de traitement. En conséquence, la minocycline est un médicament utile dans le traitement de la lèpre, et des études pour déterminer son efficacité en combinaison avec d'autres médicaments anti-lépreux, différents dosages et des administrations espacées, ainsi que ses effets sur la réaction lépreuse, devraient être poursuivies.RESUMEN
Se trataron 12 pacientes con tres dosificaciones de minociclina por 30 días, primariamente para detectar los efectos de las diferentes dosis sobre la viabilidad de Mycobacterium leprae, seguido por otros 5 meses de minociclina diaria para establecer la eficiencia general de la droga y la persistencia de sus efectos clínicos y antibacterianos. Posteriormente los pacientes recibieron la poliquimioterapia estándar de la WHO para la lepra multibacilar. La mejoría clínica fue notada durante el primer mes, ocurriendo mucho más temprano entre aquellos en tratamiento con 200 mg diarios de minociclina que entre aquellos tratados con 100 mg diarios de minociclina. También se observó un cambio similar en un paciente a los 11 días de haber recibido 3 dosis diarias de 100 mg de minociclina. Al final de los 6 meses, todos los pacientes mostraron mejoría clínica con una ligera reducción en el índice bacteriano (BI) y en el índice logarítmico de bacilos en las biopsias (LIB). Los efectos de la minociclina sobre la viabilidad, por la técnica de inoculación en la almohadilla plantar del ratón y por los ensayos de oxidación del ácido palmítico, fueron notados entre los días 10 a 14 de tratamiento diario y llegaron a ser más aparentes después de 30 días de tratamiento. Ambas pruebas mostraron una buena correlación. Las dosis de 200 mg diarios de minociclina no parecieron ser más eficientes que la dosis de 100 mg diarios de la misma. Las determinaciones de glicolípido fenólico-I (PGL-I) practicadas en algunos pacientes durante el primer mes de tratamiento, permanecieron positivas y no correlacionaron con los cambios en los resultados de viabilidad. Al final de 6 meses, después de 5 meses de monoterapia con 100 mg de minociclina, no se pudieron demostrar bacilos viables por la técnica de la almohadilla plantar del ratón y por la oxidación del ácido palmítico; los ensayos para PGL-I fueron todos negativos. No se observaron reacciones leprosas durante los 6 meses de terapia. Algunos efectos colaterales tolerables, mareo y malestar abdominal, ocurrieron sólo durante la primera semana de tratamiento en 2 de 12 pacientes. Se observó una pigmentación generalizada de color café que fue más intensa y de color azulgris sobre los sitios de las lesiones curadas. Los resultados de este estudio confirman los efectos tempranos de la minociclina sobre las lesiones clínicas y sobre la viabilidad de M. leprae, con sus efectos antibacterianos y clínicos definitivos después de 6 meses de tratamiento. Así, la minociclina es una droga valiosa en el tratamiento de la lepra pero debe investigarse aún más, su eficacia en combinación con otras drogas antileprosas, los niveles y frecuencia de la dosis, y sus efectos sobre la reacción leprosa.Multidrug therapy (MDT) is the recommended treatment for leprosy to minimize emergence of drug resistant strains of Mycobacterium leprae (14,22). The development of MDT was prompted by the marked increase in dapsone resistance in patients receiving dapsone monotherapy. Current World Health Organization (WHO)/MDT for multibacillary (MB) leprosy utilizes a combination of rifampin, dapsone and clofazimine (22). Of the three drugs, only rifampin is rapidly bactericidal. However, M. leprae strains resistant to rifampin (13), and possibly to clofazimine (21), have been reported. Lapses in compliance, which are especially a problem with the treatment durations required for leprosy, certainly contribute to treatment failure and relapse and, more importantly, to the development of bacterial resistance. The use of two or more rapidly bactericidal drugs in combination should shorten the duration of treatment and minimize compliance problems. Thus, there is a need for new, rapidly bactericidal drugs for the treatment of leprosy.
Recently, minocycline, an alkylated amino-tetracycline, widely used for bacterial infections and safely used for the long-term therapy of acne vulgaris, has been shown to have bactericidal activity against M. leprae in mice at dietary doses of 0.02%-0.04%. These doses give a minimum inhibitory concentration (MIC) for M. leprae of < 0.2 Mg/ml (9,10,l6) which is very much lower than plasma levels of 2-4 µ g/ml easily achievable in humans with doses of 100 mg 2 x daily (17). Furthermore, in vitro studies (5,8,18 ) also have shown that minocycline inhibited the metabolic activity of M. leprae. In human leprosy, minocycline at 100 mg/day for 3 months showed remarkable results (11,5).
We evaluated minocycline therapy for 6 months with three different dose levels during the first month. Drug efficacy was measured by clinical changes as well as changes in the bacterial index (BI) and histopathology. Antibacterial efTects were evaluated by determination of the viability of M. leprae in the mouse foot pad and palmitic acid oxidation assays, both being performed on the same biopsy suspensions to permit a comparison of these assays. In some patients, determination of serum phenolic glycolipid I (PGL-I) antigen also was performed at the time of the biopsies. Most viability assays were done during the first month of therapy to determine how quickly and which dosage levels would be most effective. Palmitic acid oxidation studies were done at the Leonard Wood Memorial Leprosy Research Center, Cebu, The Philippines, and at the G.W. Long Hansen's Disease Center, Louisiana, U.S.A. Adverse drug effects, intolerance, and the occurrence of lepra reactions were monitored.
METHODOLOGY
A total of 14 lepromatous or near lepromatous (13 LLand 1 BL) patients, 8 males (average age 27.4 years) and 6 females (average age 22.2 years), were sequentially admitted to the study. Of these, 10 were new, previously untreated patients and 4 were previously treated, relapsed patients. In the latter group, no active antileprosy treatment had been taken for at least 2 years before admission to the study.
Minocycline was administered in the following regimens: (A) 200 mg daily for 30 days, followed by 100 mg daily for another 5 months in 4 patients; (B) 100 mg daily for 30 days, followed by 100 mg daily for 5 months in 8 patients; (C) 100 mg daily on days 1, 2, 3 during the first 2 weeks, and another dosaging with 100 mg daily on days 15, 16 and 17 during the second 2 weeks, followed by 100 mg/day for 5 months in 2 patients.
All the patients would be treated with standard WHO/MDT for MB leprosy (1(>) after completing 6 months of minocycline monotherapy.
Informed consent was obtained and medical and laboratory clearances were performed before admission to the trial. Laboratory examinations included a complete hematologic examination, routine urinalysis and stool examinations; liver and kidney profile studies also were performed. All examinations were repeated at the end of the study, and during the study period when indicated.
Treatment was fully supervised for 10 patients admitted to the Eversley Childs Sanitarium. For the 4 who were outpatients, the drug was given as a weekly supply.
Clinical, bacteriological and histological examinations were performed following standard procedures established at the Center (Standard protocol for trials of combined drug regimens among lepromatous patients. Report of the first meeting of the THELEP Scientific Working Group, Geneva, 25-29 April 1977. TDR/SWG-THELEP (1)/77.3). Detailed clinical dermatologie/neurologie examinations were performed at pretherapy, day 28, and at the end of 6 months. Inpatients were seen daily during supervised drug administration; outpatients were seen weekly when they came for their medicines. Clinical changes and the occurrence of lepra reactions or adverse drug effects also were monitored at this time.
Bactériologie skin smears were taken from six skin sites before treatment and at the end of 6 months. Skin biopsies for histopathology, mouse foot pad inoculation, and palmitic acid oxidation studies were taken at pretherapy from skin sites having the greatest number of acid-fast bacilli (AFB). Subsequent biopsies were taken adjacent to the original biopsy sites or from lesions with similar characteristics as those of the biopsied pretherapy lesions. Suspensions prepared from the same biopsy were used for the mouse foot pad inoculation and palmitic acid oxidation assay to permit comparative studies of the two procedures. Histologic evaluation for changes in the granuloma and the logarithmic index of acidfast bacilli in biopsies (LIB) (19) were performed by comparing pretreatment histology and histology at 6 months.
Mouse foot pad viability studies were done as previously described using the Shepard technique (20). M. leprae suspensions from biopsies taken at pretherapy and at various intervals during the treatment period were inoculated into both hind foot pads of inbred CBA mice. Five thousand AFB were inoculated per foot pad. Most inoculations were done during the first month of treatment (days 3, 7, 10, 14, 21, 30) and then at day 180 (at 6 months). For each patient, 15 mice were used for pretreatment biopsy specimens (control), while 20 were used for isolates taken during treatment and at the end of 6 months. Harvests were performed 8-12 months after inoculation. AFB in the foot pads of individual mice were counted, and a 20-fold increase in the AFB count was considered positive growth.
Radiorespirometric studies were conducted by a slight modification of a previously described procedure (1). From each biopsy, for mouse foot pad inoculation, part of the remaining bacillary suspension was transferred to a sterile microfugc tube and ccntrifuged at 9430 x g x 10 min at 10ºC. The supernatant was discarded and the bacilli suspended in 1 ml Middlebrook 7H12 medium adjusted to pH 5.8 (with citric acid). The number of bacilli was determined microscopically. Part of the suspension was killed by immersion in a boiling-water bath for 10 min. Aliquots of 1 x 106 AFB were added to 6-ml scrcw-cap vials ("shorty vials;" Wheaton Scientific, Milleville, New Jersey, U.S.A.) containing 1 ml 7H12 medium, pH 5.8, containing 50 µg/m\ ampicillin and 5 Mg/ml amphotericin B. One microcurie of [l-14]C-palmitic acid (57 mCi/ mmole; New England Nuclear Corp., Boston, Massachusetts, U.S.A.) was added to each vial in a volume of 10 µl. Vials were placed with loose caps within wide-mouth scintillation vials (Poly-Q; Beckman Instruments, Fullerton, California. U.S.A.) containing a 2 x 4-cm strip of filter paper to which 100 M1 of 2 N NaOH had been added. The scintillation vial caps were tightened and the entire assembly was incubated at 33ºC for 1 week. The inner vials containing the bacilli were then removed, and the scintillation vials with enclosed filter strips were shipped by air to Louisiana, U.S.A., where the evolved M 14CO2 was measured by adding 5 ml liquid scintillation fluid and determining the counts per minute (cpm) in a Beckman LS-5801 liquid scintillation counter. Data are presented as mean cpm of replicate samples (N = 2-10) corrected for background activity by subtraction of the mean cpm from replicate heat-killed samples (N = 1-5).
In some patients, serum PGL-I antigen determinations as described by Cho, et al. (3,4) also were performed at the time of biopsies.
RESULTS AND DISCUSSION
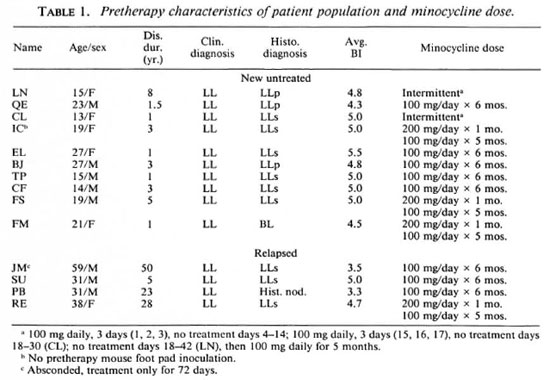
Our study is an open trial to determine the efficacy of minocycline in lepromatous leprosy. The characteristics of the patients sequentially admitted and the dose of minocycline are summarized in Table 1. One patient (JM) with relapsed leprosy absconded after receiving minocycline 100 mg daily for 2  months and was not included in the evaluation. Also, a new untreated patient (IC) with no pretherapy mouse foot pad inoculation result was not included in the evaluation of the results.
months and was not included in the evaluation. Also, a new untreated patient (IC) with no pretherapy mouse foot pad inoculation result was not included in the evaluation of the results.
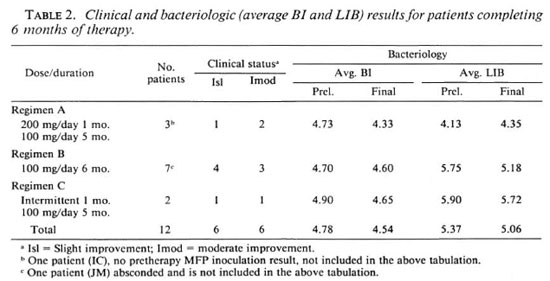
The clinical and bactériologie results after 6 months of therapy are presented in Table 2. Since the numbers of patients are limited and because no appreciable differences were observed in the clinical and bactériologie responses for new, untreated patients and previously treated, relapsed patients, the results for both groups were combined.
Clinical improvement was observed in all 12 patients completing 6 months of therapy; slight improvement in 6 and moderate-tomarked improvement in the other 6 patients. All papular and nodular lesions subsided completely. The effects of different doses during the first month is not demonstrable.
Early clinical improvement was noted during the first 30 days, subsequently becoming less dramatic. Papular, nodular and small plaque-type lesions were noted to show the earliest clinical changes. One relapsed patient receiving 200 mg daily showed fading of erythema, slight softening and slight wrinkling of the surface of the papulonodules after 3 days of treatment. In another two patients, also receiving 200 mg daily, fading of erythema and softening of the papulonodulcs with wrinkling of the overlying skin became noticeable after 7 days of treatment. Similar changes were noted after 14 days of treatment in three patients receiving 100 mg daily and in another patient, also on 100 mg daily, after 30 days of treatment. In one patient, who received only 100 mg daily for 3 consecutive days, fading of erythema, and softening and fine surface wrinkling of the papulonodulcs were noted at 14 days (11 days after the last dose of minocycline). These early changes and the more rapid responses among those receiving 200 mg daily strongly suggests an antiinflammatory effect of minocycline. Some depot effect is suggested in one patient who showed improvement 11 days after the last dose of minocycline 100 mg/day for 3 days. These recognizable changes were very encouraging to the patients, and would be an advantage of minocycline in leprosy chemotherapy.
Results of the skin smears for bacterial load (BI) (Table 2) show an overall reduction of 0.24 units after 6 months of treatment. One month of minocycline 200 mg daily or 100 mg daily or a total dosage of 600 mg (for patients treated intermittently) did not show any effect on the BI at 6 months. As can be seen, a decrease in the average BI was 0.40 units for patients on regimen A; for regimen B, 0.10 units, for regimen C, 0.25 units. None of these decreases is significant.
In an attempt to evaluate the histologic effects of treatment, the granuloma fraction and biopsy index were compared at pretherapy and after 6 months of treatment (Table 2). For the 12 patients completing the study, there was an average reduction of the granuloma fraction from 54.9% to 31.7% and an average reduction of the LIB from 5.37 to 5.06 at the end of 6 months.
No reactional episode occurred during the 6 months of minocycline therapy, again strongly suggesting an antiinflammatory effect of minocycline. This was further confirmed by our observation of the occurrence of erythema nodosum leprosum (ENL) in 6 of the 12 patients after the institution of the standard WHO/MDT chemotherapy. Mild ENL (+/++ ) developed in 4 patients (3 weeks, 5 months, 6 months and 8 months after MDT was started), and moderate-tosevere ENL (++/+ + +) occurred in 2 patients after only 2 weeks on standard WHO/ MDT chemotherapy (20).
Tolerable side effects were experienced only during the first week of treatment in 2 of 8 patients who were receiving minocycline 100 mg/day. One male patient experienced dizziness and nausea during the first week of treatment. This was remedied by taking the dose at night. The other patient was a female who experienced abdominal pain and nausea during the first 3 days of treatment, and the drug was temporarily discontinued. In this instance, the dose of minocycline was taken 3 to 4 hours after breakfast and may have caused some form of gastritis. The patient was relieved by antacid therapy. When treatment was resumed and the dose was given much closer after breakfast, no similar side effects were experienced. None of the 3 patients who received 200 mg/day, taken by 2 patients (1 female and 1 male) as a single dose in the morning, and by the other patient (female) as 100 mg in the morning and 100 mg at night during the first 30 days of the study, experienced any intolerance. All patients had a slight but definite dark brownish discoloration of the skin at 6 months which was more apparent among those with fair skin. The pigmentation was more pronounced and was bluish-gray over sites of subsided localized skin lesions. The pigmentation, however, was much less than that observed among patients receiving the standard MB WHO/MDT chemotherapy at 6 months.
Table 3 gives the results of the viability tests with the mouse foot pad inoculations and palmitic acid oxidation assays and serum PGL-I antigen determinations at various intervals during the treatment period as well as clinical evaluations for the 12 patients completing the study.
All pretherapy mouse foot pad inoculations were highly positive except in one new, untreated, histologically confirmed lepromatous patient. This patient (EL) had a pretherapy BI of 5.5 and, by chance, the proportion of viable organisms in the biopsy specimen may have been low.
For three patients who received 200 mg/ day for 1 month, a slight decrease in viability was observed in 2 patients after 14 days of treatment. At 30 days of treatment, 1 showed the absence of detectable viable organisms and 2 patients showed much reduced viability. After 5 months of minocycline 100 mg/day, no isolates had detectable viable organisms.
Among 7 patients on 100 mg/day for 1 month, a significant decrease in viability became detectable as early as 10 days after treatment in 1 of 4 patients who had mouse foot pad inoculation. After 14 days of treatment, in 3 of 6 patients with mouse foot pad inoculation, isolates were no longer viable and a much reduced viability was observed in 2 patients. After 30 days of treatment in 6 patients and 42 days of treatment in 1 patient (QE), no viable organisms were detected in 6 and very much reduced viability was detected in only 1 patient. After 5 more months of daily minocycline 100 mg/day, all isolates were nonviable.
For two patients who received minocycline 100 mg daily on days 1, 2, and 3, no effects on viability were detected from biopsy specimens taken at day 4 (after 3 days of minocycline 100 mg daily), day 7 and day 14 (4 days and 11 days, respectively, after the last dose of minocycline). However, at days 30 and 42 (13 and 25 days after the last dose of another 3 consecutive days of minocycline 100 mg/day given on days 15, 16 and 17) biopsy specimens from both patients showed a slight reduction in viability, suggesting a possible depot effect of minocycline. After daily dosages of minocycline 100 mg/day from day 31 to day 180 in one patient and from day 43 to day 180 in the other patient, no viable M. leprae could be detected in the biopsy specimens.
The results of radiorespirometric monitoring done on organisms for mouse foot pad inoculation at various intervals during treatment are also presented in Table 3. An arbitrary designation of a "negative" result in radiorcspiromctry was taken to be a mean cpm of zero or less than zero (occurs when heat-killed controls give a slightly higher reading than test samples) or when the standard deviation of the mean overlapped zero.

In all cases radiorcspiromctric values declined with treatment, although there was not always an absolute correlation with the mouse foot pad data. In most cases a sharp decline in mouse foot pad infectivity between two time points also was reflected in the radiorcspiromctric values. This relationship was also observed in clinical trials of sparfloxacin (1) and clarithromycin (2) but was not evident with a trial of fusidic acid (7).
Radiorespirometric measurement of the oxidation of palmitic acid to carbon dioxide is currently the only metabolic viability assay which has withstood the scrutiny of double-blind evaluation as a primary drugscreening assay for leprosy (6). The Buddemeyer-type assay was employed for these studies due to its greater sensitivity (compared to the BACTEC system) in terms of the minimum AFB requirement. A few patients with very high bacillary yields were evaluated by both the BACTEC and Buddemeyer assays with essentially identical results (data not shown).
An obvious advantage of radiorespirometry is the generation of quantitative data within 1 week of biopsy in contrast to the 6 to 8 months' incubation typically used to detect growth of M. leprae in the mouse foot pad. As demonstrated in this study, a liquid scintillation counter need not be present on site since the filters can easily be mailed to a distant laboratory for determination of the cpm. One limitation is that biopsies must have a minimum BI of 4 to 5 in order to obtain sufficient bacilli. Another disadvantage is the possibility of contamination with other microorganisms. This problem was rarely encountered (1 of 61 biopsies processed on site at LWM) and it appears that the addition of only ampicillin and amphotericin B is highly satisfactory for contamination control.
In 6 patients (3 on minocycline 200 mg/ day and another 3 on minocycline 100 mg/ day), serum PGL-I antigen determinations were performed at pretherapy, at 30 days, and at 6 months, and in some patients during the first month (Table 3). All pretherapy determinations were positive. At 30 days only one patient had become PGL-I negative and at 6 months all samples were negative. Positivity to PGL-I antigen during the first month showed poor correlation with the mouse foot pad inoculation and palmitic acid oxidation results. Apparently, PGL-I antigen determination is not sensitive enough to show reductions in viability as observed in the mouse foot pad and palmitic acid oxidation assays. At day 14, in one patient (CF) PGL-I antigen was still positive, while results of palmitic acid oxidation was much reduced and the mouse foot pad harvest was negative. At day 30, three patients with negative mouse foot pad harvests and much reduced radiorcspiromctric results were still positive to PGL-I antigen. Also, in another two patients PGL-I antigen was still positive while the mouse foot pad inoculation and palmitic acid oxidation assays were much reduced. Thus, PGL-I antigen is still measurable for some period of time after viability can no longer be detected by the mouse foot pad inoculation technique and the palmitic acid oxidation assay.
From the above results, a daily dose of 200 mg is no more effective than a daily dose of 100 mg as observed in viability studies, using mouse foot pad inoculation and the palmitic acid oxidation assay, during the first 30 days of treatment. In fact, more significant reductions in viability were observed among those on minocycline 100 mg/day. However, this observation is based on only 3 versus 7 patients, and may be a chance occurrence. A larger patient population is obviously needed to investigate this observation further.
In our attempt to study possible depot effects using intermittent dosaging, some reduction in the viability of M. leprae was observed in both the mouse foot pad system and by the palmitic acid oxidation assay in the two patients under this regimen with only two pulsed doses. However, we were able to observe some reduction in viability after pulsed dosaging, confirming favorable results obtained in mouse studies using intermittent dosaging (12). More intermittent dosages in a larger patient population would permit a better evaluation of intermittent dosaging.
Acknowledgment. We wish to acknowledge the excellent technical assistance given by the stafT of the clinical and laboratory branches of the Leonard Wood Memorial with special mention to Mrs. Cecilia Alerta, nurse of the clinical branch, Mrs. Rhea Fajardo, of the vivarium section; Mrs. Manucla Luisa P. Franzblau, Mrs. Elna Nunez and Mrs. Paulina Munalcm of the laboratory branch, and Mrs. Loida Gabiana for computer and clerical help. Also, we thank the Evcrsley Childs Sanitarium, the medical and technical staff for their support. Lastly, we wish to thank the Lederle Laboratories Division Cyanamid Philippines, Inc., for some supply of minocycline capsules.
This research was supported under Grant #5600 G 00 010100, program in Science and Technology Cooperation, Office of the Science Advisor, USAID, and the Leonard Wood Memorial, American Leprosy Foundation.
REFERENCES
1. CHAN, G. P., GARCIA-IGNACIO, B. Y., CHAVEZ, V. E., LIVELO, J. B., JIMENEZ, C. L., PARRILLA, M. L. R. and FRANZBLAU, S. G. Clinical trial of sparfioxacin for lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)61-65.
2. CHAN, G. P., GARCIA-IGNACIO, B. Y., CHAVEZ, V. E., LIVELO, J. B., JIMENEZ, C. L., PARRILLA, M. L. R. and FRANZBLAU, S. G. Clinical trial of clarithromycin for lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)515-517.
3. CHO, S.-N., HUNTER, S. W., GELBER, R. H., REA, T. H. and BRENNAN, P. J. Quantitation of the phenolic glycolipid of Mycobacterium leprae and relevance to glycolipid antigencmia in leprosy. J. Infect. Dis. 153(1986)560-569.
4. CHO, S.-N., SHIN, J. S., CHOI, I. H., RUN, S. H., KIM, D. I. and KUN, J. D. Detection of phenolic glycolipid-I of Mycobacterium leprae and antibodies to the antigen in sera from leprosy patients and their contacts. Yonsci Med. J. 29(1988)219-224.
5. FRANZBLAU, S. G. Drug susceptibility testing of Mycobacterium leprae in the BACTEC 460 system. Antimicrob. Agents Chemother. 32(1989)2115-2117.
6. FRANZBLAU, S. G., BISWAS, A. N., JENNER, P. and COLSTON, M. J. Double-blind evaluation of BACTEC and Buddcmcycr-typc radiorcspiromctric assays for in vitro screening of anti leprosy agents. Lepr. Rev. 63(1992)125-133.
7. FRANZBLAU, S. G., CHAN, G. P., GARCIA-IGNACIO, B. Y., CHAVEZ, V. E., LIVELO, J. B., JIMENEZ, C. L., PARRILLA, M. L. R., CALVO, R. F., WILLIAMS, D. L. and GILLIS, T. P. Clinical trial of fusidic acid for lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)1651-1654.
8. FRANZBLAU, S. G. and HASTINGS, R. C. Rapid in vitro metabolic screen for anti leprosy compounds. Antimicrob. Agents Chemother. 31(1987)780-783.
9. GELBER, R. H. Minocycline studies in mice of a promising agent for the treatment of Leprosy. Int. J. Lepr. 54(1986)722-723.
10. GELBER, R. H. Activity of minocycline in Mycobacterium leprae- infected mice. J. Infect. Dis. 156(1987)236-239.
11. GELBER, R. H., FUKUDA, K., BYRD, S., MURRAY, L. P., SIN, P., TSANG, M. and REA, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. J. 304(1992)91-92.
12. GELBER, R. H., SIN, P., TSANG, M., ALLEY, E. and MURRAY, L. P. Effect of low-level and intermittent minocycline therapy on growth of M. leprae in mice. Antimicrob. Agents Chemother. 35(1991)992-994.
13. JACOBSON, R. R. and HASTINGS, R. C. Rifampin resistant leprosy. Lancet 2(1976)1304-1305.
14. Ji, B. and GROSSET, J-H. Recent advances in the chemotherapy of leprosy. (Editorial) Lepr. Rev. 61(1990)313-329.
15. Ji, B., JAMET, P., PERANI, E. G., BOBIN, P. and GROSSET, J. N. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in the treatment of lepromatous leprosy. J. Infect. Dis. 168(1993)188-190.
16. Ji, B., PERRANI, E. G. and GROSSET, J. H. Effectiveness of clarithromycin and minocycline alone and in combination against Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
17. MACDONALD, H., KELLY, R. G., ALLEN, E. S., NOBE, J. F. and KANEGIS, L. A. Pharmacokinetic studies on minocycline in man. Clin. Pharmacol. Ther. 14(1973)852-861.
18. RAMASESH, N., KRAHENBUHL, J. L. and HASTINGS, R. C. In vitro effects of antimicrobial agents on Mycobacterium leprae in mouse peritoneal macrophages. Antimicrob. Agents Chemother. 33(1989)657-662.
19. RIDLEY, D. S. and HILSON, G. R. F. A logarithmic index of bacilli in biopsies. Int. J. Lepr. 35(1967)184-186.
20. Shepard, C. C. Multiplication of M. leprae in the foot pad of the mouse. Int. J. Lepr. 30(1962) 291-306.
21. WARNDORFF-VAN DIEPEN, T. Clofazimine-resistanl leprosy, a case report. Int. J. Lepr. 50(1982 )139-142.
22. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Scr. 675 .
1. M.D., M.P.H., Senior Clinical Consultant.
2. M.D., Acting Chief, Clinical Research Branch.
3. D.V.M., Chief, Vivarium.
4. M.D., Pathologist.
5. Ph.D., Scientific Director, Leonard Wood Memorial Center, P. O. Box 727, Cebu City, The Philippines.
6. Ph.D., Chief, Pharmacology Research Department, Laboratory Research Branch, GWL Long Hansen's Disease Center at Louisiana State University, P. O. Box 25072, Baton Rouge, Louisiana, U.S.A.
Received for publication on 25 May 1994.
Accepted for publication in revised form on 30 August 1994.