- Volume 63 , Number 1
- Page: 18–22
Incidence of Late lepra reaction among multibacillary leprosy patients after MDT
ABSTRACT
Multidrug therapy (MDT) recommended by the World Health Organization (WHO) had been administered in 1982 to a cohort of multibacillary (MB) leprosy patients. Treatment was administered for a minimum period of 2 years or until skin-smear negativity for acid-fast bacilli was achieved (whichever was later). Among 980 MB leprosy patients who completed treatment, 11 patients (1.1%) experienced lepra reactions during surveillance. Probable predictive factors are discussed. The incidence of lepra reaction seemed to be three times more common in borderline (BL) leprosy than in lepromatous (LL) leprosy. The majority of these events occurred during the first 3 years of surveillance. All of these episodes were treated withsteroids without antileprosy chemotherapy. None of these patients was confirmed as experiencing a relapse during the subsequent period of surveillance.RÉSUMÉ
La poychimotherapie (PCT) recommandée par l'Organisation Mondiale de la Santé (OMS) a été administrée en 1982 à une cohorte de patints lépreux multibacillaircs (MB). Le traitement a été administré pour une période minimale de deux ans ou jusqu'à négativation des frottis cutanés pour les bacilles acido-résistants (quel que soit ce qui arrivait en dernier lieu). Parmi 980 malades de la lèpre qui ont terminé le traitement, 11 malades (1.1 %) ont eu une réaction lépreuse durant la surveillance. Des facteurs prédictifs probables sont discutés. L'incidence de la réaction lépreuse semblait trois fois plus commune dans la lèpre borderline (BL) que dans la lèpre lépromateuse (LL). La majorité de ces épisodes est survenue durant les trois premières années de la surveillance. Tous ces épisodes ont été traités avec des stéroi'des sans chimiothérapie antilépreuse. On n'a confirmé de rechute pour aucun de ces patients durant la suite de la période de surveillance.RESUMEN
En 1982 se adiminstró la poliquimioterapia (PQT) recomendada por la Organización Mundial de la Salud (OMS) a un grupo de pacientes con lepra multibacilar (MB). El tratamiento fue administrado por un periodo mínimo de 2 años o hasta que los extendidos de linfa cutánea fueron negativos para bacilos ácido-resistentes (lo que haya ocurrido más tarde). De los 980 pacientes con lepra MB que completaron su tratamiento, 11 pacientes (1.1%) desarrollaron reacciones leprosas durante el periodo de seguimiento. Se discuten los probables factores predictivos. La incidencia de la reacción leprosa pareció ser 3 veces más común entre los pacientes con lepra intermedia (BL) que entre los pacientes con lepra lepromatosa (LL). La mayoría de estos eventos ocurrió durante los primeros 3 años de seguimiento. Todos estos episodios fueron tratados con esteroides sin terapia antilcprosa. Ninguno de estos pacientes tuvo una recaída confirmada durante el periodo subsecuente de seguimiento.Gone are the days when a leprosy patient was expected to take treatment for life. The introduction of a multidrug therapy (MDT) regimen recommended by the World Health Organization (WHO) (8) has shortened the duration of therapy in leprosy, especially multibacillary (MB) leprosy. It has been demonstrated that Mycobacterium leprae are present in deeper tissues (3,6) of leprosy patients even after varying periods of chemotherapy (1,2.4,6,7), Chemotherapy can only effect bacterial killing. Residual bacteria and/or bacterial fragments still must be cleared by the immune system. Hence, leprosy patients, even though declared cured, might still be prone to complications such as lepra reactions, and it is possible that relapse may manifest itself along with the lepra reaction.
In this study lepra reactions encountered in MB leprosy patients after MDT, i.e., during surveillance, are presented.
MATERIALS AND METHODS
At the Schieffelin Leprosy Research and Training Center, Karigiri, South India, a cohort of 1067 MB leprosy patients (on record during the year 1982) were given WHO/ MDT for a minimum period of 2 years or until smear negativity, whichever was later; 980 patients successfully completed the prescribed MDT and were on surveillance, being reviewed every third month. A detailed assessment of each patient was done annually. The annual dropout rate was 2.3% (death and migration).
A few interesting clinical events were encountered during surveillance. For example, one of the MB leprosy patients successfully completed MDT in 1984. During routine surveillance a year later, he reported to the physician with prominent skin lesions and ulnar neuritis. His symptoms were acute in onset, and skin smears were negative for acid-fast bacilli (AFB). Under such circumstances the physician has to decide the line of management, whether it is a relapse needing antileprosy treatment or an episode of lepra reaction.
RESULTS
During the surveillance period (1984 to 1993), 11 patients (1.1%) presented with reactional episodes. A majority of these 11 patients had received dapsonc (DDS) monotherapy for more than 2 years (Table 1). Among those who had received DDS monotherapy for more than 2 years prior to MDT, 0.82% developed late lepra reaction. Of those patients receiving DDS monotherapy for up to 2 years, 3.2% developed a late lepra reaction.
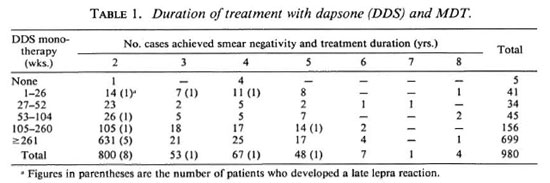
Thus, the incidence rate was four times greater among those who had received DDS monotherapy for up to 2 years prior to MDT (Fisher's exact test, p = 0.04). The incidence rate for a late lepra reaction was 2 to 4 times greater among those who had received DDS monotherapy and/or MDT for up to 2 years. The incidence rate of late lepra reaction was 14 times greater among those who were smear positive at the start of MDT and who had received DDS monotherapy for up to 2 years (Table 2).
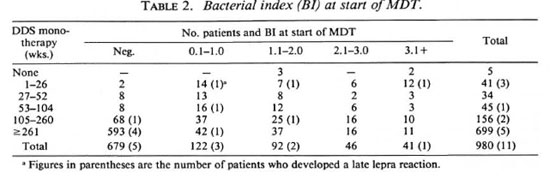
About 7% of our MB leprosy patients suffered from lepra reaction during MDT. Among the 11 patients with late lepra reaction, only 3 of them had lepra reaction during MDT. Among the lepromatous (LL) patients on surveillance, 0.7% developed a late lepra reaction; whereas 2.3% of the borderline lepromatous (BL) patients on surveillance developed a late lepra reaction (Fisher's exact test, p = 0.04).
Only 0.2% of the patients presented with manifestations limited to skin lesions, i.e., raised leprosy patches or erythema nodosum leprosum (ENL) nodules; 0.7% of the patients had only neuritis (Table 3); 0.2% had both skin and nerve involvement. Overall, 9 out of 11 patients (0.9%) had nerve involvement during these episodes. The majority of the neuritis seen occurred among BL patients.
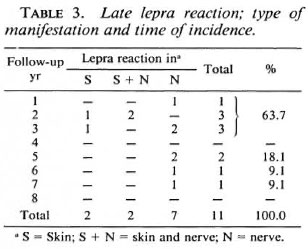
Seven out of 11 (63.7%) reactions occurred within the first 3 years of surveillance (Table 3). Skin manifestations occurred only during the first 3 years; whereas neuritis was encountered even during the seventh follow-up year. The incidence rate of late lepra reaction (from the point of completion of MDT) was higher during the initial 3 years of surveillance (Fig. 1) These patients had received MDT for varying periods (2 to 8 years). The incidence of all lepra reactions from the point of commencement of MDT is shown in Figure 2. The incidence was high during the first year and then there was a marked decline (period of treatment). The incidence rate was very minimal from the fourth year onward when more than 85% of the patients were on surveillance.
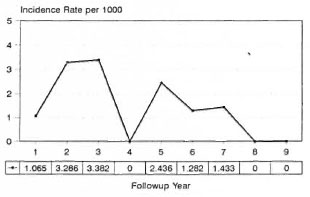
Fig. 1. Incidence of late lepra reaction after MDT.
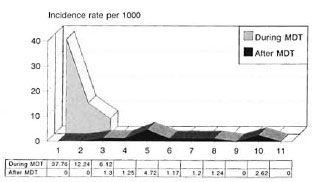
Fig. 2. Incidence of lepra reaction in MB patientsduring and after MDT.
All patients were bacteriologically negative for AFB. One patient once showed a few AFB in the skin smear 3 years after the rcactional episode.
Management and outcome. Steroids were administered for periods ranging from 4 weeks to 16 weeks. All skin manifestations responded favorably (Fig. 3). Out of 9 patients with neuritis, 4 recovered without any loss of nerve function, 1 patient had visible deformity, and 4 had residual muscle weakness. All 11 patients were follow up until 1993, and none of them was confirmed as a case of relapse.
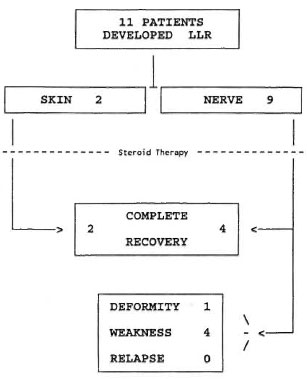
Fig. 3. Management and outcome of late lepra reaction (LLR) after release from treatment.
Observations.
1. The proportion of BL patients exhibiting late lepra reaction was three times greater tha n that of LL patients.
2. There was no difference in the incidence rate of late lepra reaction in smearpositive and-ncgative groups. However, the incidence rate of late lepra reaction was 14 times more among those who were smear positive at the start of MDT and who had received DDS monotherapy for 2 years or less.
3. The incidence rate of late lepra reaction was 2 to 4 times greater among those who had received DDS monotherapy and/or MDT for up to 2 years.
4. Reactional episodes presenting with skin manifestations occurred only during the first 3 years of surveillance.
5. Neuritis was encountered even at the seventh year of surveillance.
6. Skin-smear examinations at the time of the clinical events reported were negative for AFB.
7. There were no relapses among this cohort of 980 MB leprosy patients followed up.
DISCUSSION
MDT has been implemented with a wider coverage than ever before. The duration of treatment has been reduced to a great extent, especially in MB leprosy. At the end point of treatment, the clinical activity of disease ceases and the bacterial load is reduced remarkably so that routine skin smears are not able to demonstrate the presence of M. leprae. When such "cured" leprosy patients reveal clinical signs of active leprosy, the first possibility usually considered is relapse.
MDT with an effective combination of drugs is known to achieve almost 100% bacterial killing in leprosy, but complete antigenic clearance may not be assured within this "short" period. Hence, these patients may be prone to develop complications, i.e., lepra reaction, especially during the initial period of surveillance.
Therefore, all such "suspected relapses" were given a therapeutic trial with steroids. The reported clinical events responded to the steroid therapy. None of these patients had persistent clinical disease activity and skin smears were persistently negative for AFB during the subsequent period of surveillance. Hence, these clinical events were considered to be episodes of lepra reactions.
The group of patients previously untreated or less treated (up to 2 years of DDS and/ or MDT) seem to have a higher risk of late lepra reaction. The pattern of the incidence rate of lepra reactions after starting MDT (Fig. 2) shows that there was a marked decline from the third year onward. These episodes would have occurred even if MDT was continued.
Although the incidence of such events is small, it is still important to prevent disability and patients should be educated to undergo regular follow-up examinations. A reactional episode should be identified at an early stage for appropriate management.
The incidence rate of late lepra reaction during the first 3 years of surveillance is about 2.6 per 1000 person years of surveillance (Fig. 1). The risk declines during the subsequent years. There were no relapses during 7123 person years of follow up among this cohort of 98 MB leprosy patients followed up.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR). Wc extend our sincere thanks to the World Health Organization for the financial support provided for this project. We thank Mr. P. Samuel (project coordinator), Mr. Raja Samuel (assistant statistician), Mr. Lewis Kumar (office secretary), and all our staff who contributed their efforts and skills for the successful implementation of the project.
REFERENCES
1. HAIMANOT, R. T., MSHANA, R. N. , MCDOUGALL, A. C. and ANDERSEN, J. G. Sural nerve biopsy in leprosy patients after varying periods of treatment: histopathological and bacteriological findings on light microscopy. Int. J. Lepr. 52(1984)163-170.
2. HAIMANOT, R. T., MSHANA, R. N. , MCDOUGALL, A. C, ANDERSEN, J. G., and BELEHU, A. Muramidasc (lysosomc) findings in sural and radial ncrv biopsies in leprosy patients after varying periods of treatment. Int. J. Lepr. 53(1985)238-246.
3. HOLLA, V. V., KENETKAR, M. V., KOLHATKAR, M. K. and KULKARIN, C. N. Leprous synovitis; a study of fifty cases. Int. J. Lepr. 51(1983)29-32.
4. KATOCH, V. M., KATOCH, K, RAMANATHAN, U., SHARMA, V. D., SHIVANNAVAR, C. T., DATTA, A. K. and BHARADWAJ, V. P. Effect of chemotherapy on viability of Mycobacterium leprae as determined by ATP content, morphological index and FDA-EB fluorescent staining. Int. J. Lepr. 57(1989)615-621.
5. PANNIKAR, V., JESUDASAN, K., VIJAYAKUMARAN, P. and CHRISTIAN, M. Relapse or late reversal reaction? Int. J. Lepr. 57(1989)526-528.
6. SRINIVASAN, H., RAO, K. S. and IYER, C. G. S. Discrepancy in the histopathological features of leprosy lesions in the skin and peripheral nerve. Lepr. India 54(1982)275-282.
7. WATERS, M. F. R., REES, R. J. W., MCDOUGALL, A. C. and WEDELL, A. G. M. Ten years of dapsonc in lepromatous leprosy: clinical, bacteriological and histological assessment and the finding of viable leprosy bacilli. Lepr. Rev. 45(1974)288-298.
1. P. Vijayakumaran, M.B.B.S., D.P.H., Associate Epidemiologist.
2. N. Manimozhi, M.B.B.S., D. H. E., Senior Medical Officer.
3. K. Jesudasan, M.B.B.S., D. T. P. H., Ph.D. (London), Head, Branch of Epidemiology and Leprosy Control, Schieffelin Leprosy Research and Training Center, Karigiri 632106, India.
Received for publication on 29 July 1994.
Accepted for publication in revised form on 26 September 1994.