- Volume 63 , Number 1
- Page: 98–99
Acid-Fast Bacilli f rom former leprosy regions in Coastal Norway showing PCR positivity for Mycobacterium leprae
To the Editor:
Noncultivable acid-fast bacilli (NC-AFB) have been found in sphagnum samples collected in 1976 and 1977 in former leprosy regions of coastal Norway (4). Attempts to differentiate these NC-AFB have been made using white and nude mice and nine-banded armadillos. The results have indicated that the isolated microorganism could not be distinguished from Mycobacterium leprae (1). In another study carried out in a leprosy region of Bombay, M. leprae were found in polluted soil (2).
The second study in coastal Norway in 1986 succeeded in isolating NC-AFB with M. leprae- specific phenolic glycolipid-I in their cell wall. Experiments with nude mice indicated that these microorganisms had lost their pathogenicity (3). To clarify whether these microorganisms possess the same molecular genetic pattern as M. leprae, a third examination was carried out.
In October 1992, 14 samples of sphagnum and musci vegetation were collected on the island of Sotra near Bergen, Norway, in the same sphagnum bog where in both 1976 and 1986 NC-AFB were found (3,4). The samples were collected with sterile gloves and kept in a refrigerator until processed. Sterile plastic syringes were filled with approximately 100 cc of the grey layer of the sample, compressed, fluid collected, and centrifuged at 8000 x g x 30 min. To remove the humic acid and to reach a neutral reaction, the sediment was placed on a membrane filter, pore size 0.2 µ m, and washed 5 times with 100 ml of 1 M Tris buffer pH 8.5. The sediment was removed from the filter using 100 ml of 1 M Tris buffer pH 7.0 pressed in the opposite direction, the fluid ccntrifuged as above, and the sediment suspended in 0.5 ml of the last buffer solution. Smears were made, stained by the Ziehl-Neelsen (ZN) method for AFB, and the fluid used for further examination.
Lysis of the sample. In 1.5-ml screw-cap reaction cups (Eppendorf) containing 100 µ l of fluid, 50 µ l Proteinase K (in Tris buffer pH 8.5) and 50 µ l Twecn 20 were added and covered with 50 µ l ultra-light mineral oil (Sigma) to prevent evaporation, and incubated in a water bath for 18 hr at 60ºC. Thereafter, the samples were incubated at 97ºC for 15 min to stop Proteinase K activity. The samples were frozen at - 20ºC until used.
Primers for polymerase chain reaction (PCR). The primers, found previously to be specific for M. leprae in a detailed comparison using M. leprae and 42 other mycobacterial species (5), were synthesized on an Applied Biosystems DNA synthesizer. The primers, SOD/I and SOD/II, were used to amplify a 242-base-pair fragment of the gene encoding for superoxide dismutase of M. leprae. The oligonucleotide primers have the following sequences: primer SOD/I (308) = 5'3'ATTGATGAAACGTTTGGGTC; primer SOD/II (530-550) = 3'5'TTGACGTAATCCGCCTTGAC.
Amplification of DNA by PCR. Twentyfive µ l of the samples to be analyzed was incubated in a microfuge tube containing 25 µ l of the reaction mixture [10 mM Tris-HC1 (pH 9.6), 50 mM NaCl, 0.01% gelatin, 12.5 mM MgCl2, 200 µ l of each of the oligonucleotide primers, 1 mM each of dATP, dCTP, dGTP, dTTP, and 2.5 units of Taqpolymerase]. Negative (with distilled water) and positive (with purified armadillo-derived M. leprae) samples were used as the controls. The reaction mixtures were covered with 25 µ l of ultra-light mineral oil (Sigma). Cycles of amplification consisted of a 2-min denaturation at 94ºC, a 2-min annealing step at 59ºC, and a 3-min elongation step at 72ºC performed in a programmed DNA Thermal Cycler (automated Pharmacia LKB - Gene ATAQ Controller) and repeated 32 times. The samples were analyzed by electrophoresis (5).
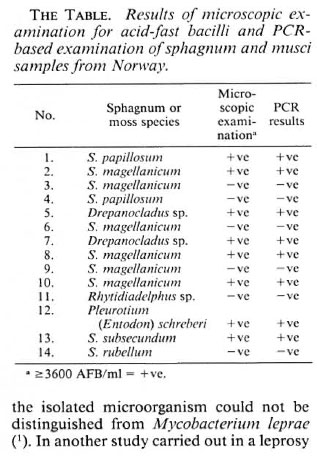
Of 14 samples, 8 were ZN-positive and the same samples revealed specific positivity for M. leprae (The Table). The Figure shows the results with 6 selected positive samples, armadillo-derived M. leprae in two concentrations (104 and 102) and one control. In the previous experiments, the low pH, caused by the presence of humic acid, hindered the action of Taq-polymerase (5). The modification of the procedure by washing with Tris buffer (pH 8.5) enabled the use of the PCR technique in sphagnum and musci samples.
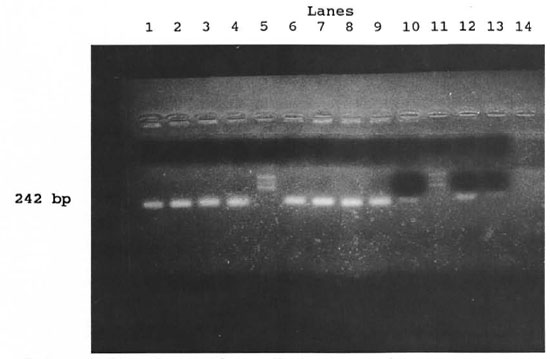
The figure. Positive reactions with M. leprae -specific primcrs in samples ofsphagnum and musci vegetation. Lanes from the left: Samples nos. 1 (lane 1), 2 (lane 2), 5 (lane 3), 7 (lane 4), marker (lane 5), samples nos. 8(lane 6) and 10 (lane 7). Positive controls: ML (lane 8), ML (lane 9), ML-dil. (lane 10), marker (lane 11), ML-dil. (lane 12), ML-dil. (lane 13). Negative control (lane 14). ML = armadillo-derived M. leprae 4.4 x 104/m1; ML-dil. = armadillo-derived M. leprae 4.4 x 102/ml.
The results confirmed that the NC-AFB present in sphagnum and musci vegetation possess the same fragment of the gene encoding for superoxide dismutase as M. leprae.
- Hamdy Mahfous Mostafa, M.D.
Faculty of Medicine
University of Assiut, Egypt
- Jindrich Kazda, M.V.D., Ph.D.
Professor
Institut fur Experimentelle Biologic und Medizin
D-23845 Borstel, Germany
- Lorentz M. Irgens, M.D.
Professor
Faculty of Medicine
University of Bergen, Norway
- Hans Gerd Luesse, Dr.Agr.
University Onsabruck, Germany
REFERENCES
1. KAZDA, J. Occurrence of noncultivable acid-fast bacilli in the environment and their relationship to M. leprae. Lepr. Rev. 52 Suppl. 1(1981)85-91.
2. KAZDA, J., GANAPATI, R., REVANKAR, C, BUCHANAN, T. M., YOUNG, D. B. and IRGENS. L. M. Isolation of environment-derived M. leprae from soil in Bombay. Lepr. Rev. 57 Suppl. 3(1986)201-208.
3. KAZDA, J., IRGENS, L. M . and KOLK, A. Acid-fast bacilli found in sphagnum vegetation of coastal Norway containing M. leprae -specific phenolic glycolipid-I. Int. J. Lepr. 58(1990)353-357.
4. KAZDA, J., IRGENS, L. M . and MULLER, K. Isolation of noncultivable acid-fast bacilli in sphagnum and moss vegetation by foot pad technique in mice. Int. J. Lepr. 48(1980)1-6.
5. LUESSE, H. G. Untersuchungen zum Nachweis von M. leprae mit der Polymarase-Kettenreaktion. Gottingener Beitr. Land-und Forstwirtschaft in den Tropen und Subtropen. Hefte 78(1993)1-139.
Reprint requests to Dr. Kazda.