- Volume 63 , Number 1
- Page: 105–9
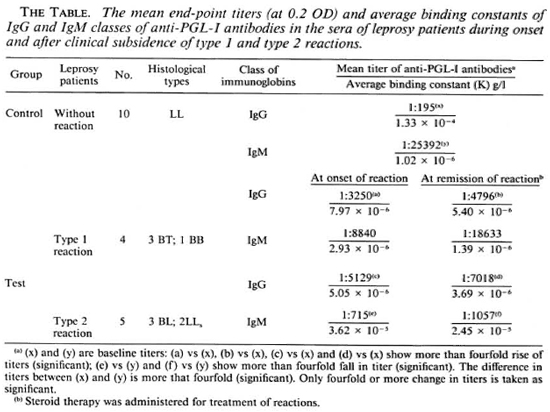
Enhanced response of serum IgG class of anti-PGL-I antibodies in leprosy patients during onset and following clinical remission of type 1 and type 2 reactions
To the Editor:
Phenolic glycolipid-I (PGL-I) contains a trisaccharide, unique to Mycobacterium leprae (2). Reports are available on the response of serum anti-PGL-I antibodies in untreated and successfully treated leprosy patients (11) as well as in contacts (15). However, little information is available on the response of serum anti-PGL-I antibodies during lepra reaction and after its remission following steroid therapy. There is no study on the binding of anti-PGL-I antibodies for its ligand.
The micro-ELISA technique is often used to estimate the serum levels of anti-PGL-I antibodies (6). The affinity dependence of these assays is marked when antigen of low epitope density is employed (13). The number of epitopes in the PGL-I molecule is perhaps limited. Monoclonal IgG to PGL-I reacts exclusively with the distal 3,6-di- O -methyl- β -D-glucopyranose(5).
Keeping this in mind, we have designed this study aiming at quantifying serum levels of IgG and IgM classes of anti-PGL-I antibodies employing the micro-ELISA technique (6) in 9 patients (5 males and 4 females between 21 and 45 years of age) at the onset of type 1 and type 2 reactions as well as following clinical remission after steroid therapy and compared their sera antibody levels with those in 10 lepromatous leprosy (LL) patients without reaction (6 males and 4 females between 25 and 45 years of age).
To estimate the binding constants (K) of anti-PGL-I antibodies in the sera of these patients we compared their scrum titers with that of a mouse monoclonal (MC-1433-S) (WHO) containing a known amount of the IgG1 subclass of the specific antibody. Sonicated PGL-I antigen (provided by Dr. P. J. Brennan), HRPO conjugated rabbit antihuman IgM ( µ -chain specific) and anti-human IgG (γ-chain specific) antisera (Capped Laboratories, U.S.A.) were employed. Each micro-ELISA plate included a negative control (a pooled serum from 9 healthy human volunteers) and a positive control (a pooled serum from 10 LL patients without reaction). Each test serum (10 LL patients without reaction and 9 patients at the onset and after remission of reaction) was serially diluted and allowed to react with 0.1 µ g PGL-I which had been immobilized on a U-bottom polystyrene plate (Dynatech Laboratory, U.S.A.) (6). Optical densities (OD) were recorded at 492 nm. Graphs were constructed by plotting the OD against log10 serum dilutions. The end point titer of a test serum was that dilution of the serum which showed 0.2 OD (The Figure). K of IgG1-anti-PGL-I antibodies was found by dividing the concentration of IgG1 anti-PGL-I antibodies in the undiluted monoclonal (0.0259 g/1) by the end point titer (1:3162) of the monoclonal. The K of the IgG and IgM classes of anti-PGL-I antibodies in the above test sera (10 from LL patients without reaction and 18 sera from 9 reactional patients at onset and remission of type 1 and type 2 reactions) were determined by dividing 0.0259 g/1 by the respective end point titers.
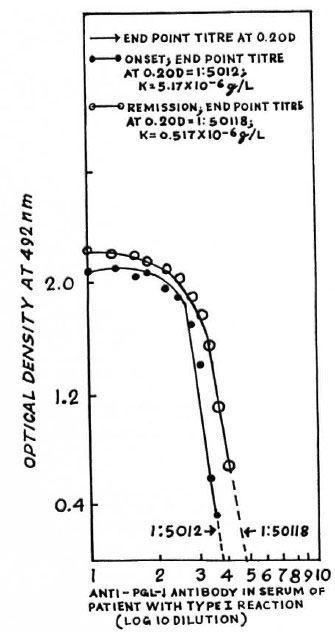
The figure. Typical titration curves of IgM anti-PGL-I antibodies in serum of a leprosy patient (AK) at onset and remission of type 1 reaction. Optical densities (OD) at 492 nm have been plotted against thevarious logo dilutions of the paired sera. End-point titers at 0.2 OD have been determined by extrapolation. K of IgM class anti-PGL-I antibodies has beendetermined by dividing 0.0259 g/1 by the respective end-point titers. The computed values of the binding constants of the IgM class of the specific antibodies are not totally accurate because the calculations assumed that all IgM molecules in the test sera were bound to PGL-I, given in excess in each well, especially when the end-point dilutions were high (1:5012 and 1:50,118). Secondly, various classes of specific antibodies do notcompete with each other for PGL-I given in excess.
The end-point titers of the various sera were grouped, and the mean end-point titers were determined. The mean end-point titer of IgM-anti-PGL-I antibodies in 10 LL patients without reaction was 1:25,392, which was higher than that of the IgG antibodies (1:195) in the sera of these patients (The Table). During onset of type 1 reaction the mean serum titer of IgM-anti-PGL-I antibodies was 1:8840, which was less than that (1:25,392) in the LL patients without reaction. After clinical remission of type 1 reaction, the mean titer of IgM-specific antibodies increased to 1:18,633 from 1:8840 found at the onset of reaction. However, both of these two mean titers (at onset and remission of type 1 reactions) were less than that (1:25,392) in the LL patients without reaction. On the other hand, the mean titers of the IgG class of anti-PGL-I antibodies in the patients' sera at the onset and remission of type 1 reaction were 1:3250 and 1:4796, respectively. Both of these titers were higher than that (1:195) in the LL patients without reaction.
During onset of type 2 reaction the mean titer of the IgM class of anti-PGL-I antibodies was 1:715, remarkably less than that (1:25,392) in the LL patients without reaction. After clinical remission of type 2 reaction the mean titer of the IgM class of the specific antibodies remained practically unchanged (1:1057). The mean titers oflgG class of anti-PGL-I antibodies in the sera of leprosy patients at onset and remission of type 2 reaction were 1:5129 and 1:7018, respectively, higher than that (1:195) found in the LL patients without reaction (The Table).
The binding constant (K) of the IgG, subclass of anti-PGL-I antibodies in the monoclonal was (0.0259/3162) g/1 = 8.19 x 10-6 g/l. We assumed that the IgG, antibodies in the monoclonal were equivalent to human IgG/IgM antibodies (g/g) with respect to their reactivity with the horseradish peroxidase conjugate. The mean K of serum IgG antibodies in the patients at the onset of both type 1 and type 2 reactions were
7.97 x 10"6 g/1 and 5.05 x 10-6 g/l, respectively, about 20-fold more than that seen in LL patients without reactions (1.33 x 10-4 g/l) (The Table). The mean K of the IgM class of antibodies in the sera of the patients at the onset of both types of reactions were 2.93 x 10-6 g/l and 3.62 x 10-5 g/1, respectively. The latter was threefold less than that (1.02 x 10-6 g/l) in the LL patients without reaction. This enhanced synthesis of the high-affinity IgG class of anti-PGL-I antibodies during onset of both type 1 and type 2 reactions as compared to that in the sera of LL patients without reaction persisted even after clinical remission of the reactions following administration of steroids (The Table).
Contradictory reports exist on the serum anti-PGL-I antibody responses during lepra reactions. Low serum levels of the IgM class of anti-PGL-I antibodies had been reported during type 2 reaction (1,9), while no consistent trend of response of any class of specific antibodies against PGL-I was found by others (14). Our study showed a decreased level of IgM-anti-PGL-I antibodies during the onset of type 1 and type 2 reactions, more so in type 2 reactions, which was evidenced by the low mean titers of scrum IgM antibodies in these reactional patients in comparison to that in the LL patients without reaction. The synthesis of IgG class of anti-PGL-I antibodies was enhanced in patients during the onset of type 1 and type 2 reactions and even increased after clinical remission of the reactions. The increase of K in the IgG class of anti-PGL-I antibodies during reaction (The Table) suggested affinity maturation of IgG antibodies, which is a T-cell-depcndent event (7-10).
Upregulation of M. leprae- specific T cells occurs during both types of lepra reactions, resulting in the active engagement of cellmediated immunity in the nerves and lesions (8) with eventual release of intracellular bacterial antigens (4,12). We postulate that the M. leprae- specific T cells, being activated during reactions, render help to the specific B cells leading to an enhanced synthesis of IgG class anti-PGL-I antibodies. The decrease of K in the IgM antibodies viz-a-viz the increase of K in the IgG antibodies during lepra reactions may be due to switching of IgM to IgG synthesis, which is a T-cell dependent event (7-10). This overproduction of IgG class anti-PGL-I antibodies during reaction compared to the predominant synthesis of IgM antibodies in the LL patients without reaction suggests that the former is a secondary response against intracellular M. leprae antigens released during reactions in the patients, who are already primed with PGL-I. Further, secondary response is known to be steroid resistant (16), which also supports the observed persistent production of IgG-anti-PGL-I antibodies in the patients who have been given steroid therapy to control reactions. Brett, et al. reported that a high antigenic load is necessary to stimulate antibody of the IgG class to the glycolipid (3). The observed high serum levels of the IgG class of anti-PGL-I antibodies in the patients after subsidence of type 2 reaction provides an explanation for the persistence of circulatory immune complexes in them long after they recover from reaction.
- Kunal Saha, M.Sc, M.B.B.S., Ph.D. (U.S.A.)
Department of Immunology
V.P. Chest Institute
Delhi, India
- Debasis Chattopadhya, M.D.
Department of Microbiology
National Institute of Communicable Diseases
Delhi, India
- Arvind Kashyup, M.B.B.S.
Department of Immunology
V.P. Chest Institute
Delhi. India
- Uma Agarwal, M.B.B.S.
School of Tropical Medicine
Calcutta, India
- Asit K. Chakrabarty, M.Sc, Ph.D.
Department of Biochemistry
University College of Medical Sciences
Delhi, India
REFERENCES
1. ANDREOLI, A., BRETT, S. J. and DRAPER, P. Changes in circulating antibody levels to phenolic glycolipid during erythema nodosum leprosum in leprosy patients. Int. J. Lepr. 53(1985)211-217.
2. BRENNAN, P. J. and BARROW, W . W . Evidence of species-specific lipid anti-antigen in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
3. BRETT, S. J., DRAPER, P., PAYNE, S. N. and REES, R. J. W. Serological activity of a characteristic phenolic glycolipid from Mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin. Exp. Immunol. 52(1983)271-2790.
4. BRITTON, W. J. Immunology of leprosy. Trans. R. Soc. Trop. Med. Hyg. 87(1993)508-514.
5. CHO, S.-N., FUJIWARA, T., HUNTER, S. W., REA, T. H., GELBER, R. H. and BRENNAN, P. J. Use of artificial antigen containing the 3,6-di- O -methyl- β -D-glycopyranosyl epitope for the scrodiagnosis of leprosy. J. Infect. Dis. 150(1984)311-322.
6. CHO, S.-N., YANAGIHARA, Y., HUNTER, S. W., GELBER, R. H. and BRENNAN, P. J. Serological specificity of phenolic glycolipid-I from Mycobacterium leprae and use in scrodiagnosis of leprosy. Infect. Immun. 41(1983)1077-1083.
7. COOPER, B. B lymphocyte differentiation. In: A course in molecular and cellular basis of immunity . Robert, M. J. and McConnel, I., eds. Oxford: Blackwell Scientific Publications, 1975, p. 197.
8. COOPER, C. L., MULLER, C, SINKHAIRARI, T. A., PRIMES, C, CHAN, J., KAPLAN, C, TOUNG, S. M. M., WEISMAN, L. L., BLOOM, B. R., REA, T. H. and MODLIN, R. L. Analysis of naturally occurring DT H reaction in leprosy by in situ hybridization. J. Exp. Med. 169(1989)1565-1581.
9. MEEKER, M. C, LEVIS, W. R., SERSEN, E., SCHULLER-LEVIS, G., BRENNAN, P. J. and BUCHANAN, T. M. ELISA detection of IgM an tibodies against phenolic glycolipid in the management of leprosy; a comparison between laboratories. Int. J. Lepr. 54(1986)530-534.
10. MILLER, J. F. A. P. Induction immune (allergic) response. In: clinical aspects of immunology. 3rd edn. Gell, P. G. H., Coombs, R. R. A. and Lachmann, P. J., eds. Oxford: Blackwell Scientific Publications, 1975, pp. 447-469.
11. MILLER, R. A., GORDER, D. and HARNISCH, J. P. Antibodies to phenolic glycolipid during long term therapy; serial measurements in individual patients. Int. J. Lepr. 55(1987)633-636.
12. SENGUPAT, U. Cell-mediated immunity in leprosy; an update. (Editorial) Int. J. Lepr. 61(1993)439-454.
13. SREWARD, M. W. Overview: introduction to methods used to study the affinity and kinetics of antibody-antigen reaction. In: Handbook of experimental immunology. vol. 1 immunochemistry. 4th edn. Weir, D. M., ed. Oxford: Blackwcll Scientific Publications, 1991, pp. 25.1-25.30.
14. TRUMAN, R. W., SHANNON, E. J. and HASTINGS, R. C. Host response to phenolic glycolipid antigens to M. leprae. (Abstract) Int. J. Lepr. 53(1985)710-711.
15. ULRICH, M., SMITH, P. G., SAMPSON, C, ZUNIGA, M., CENTENO, M., GRACIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid in contacts of leprosy patients in Venezuela: epidemiological observations and prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-408.
16. WHITTINGHAM, S., RICHLEY, J. D. and MACKAY, I. R. Factors influencing the secondary' antibody response to fiagcllin in man. Clin. Exp. Immunol. 34(1978)170-178.
Reprint requests to Dr. Kunal Saha, 45A Sova Bazar Street, Calcutta 700005, India.