- Volume 63 , Number 1
- Page: 77–85
Inflammatory bone changes in leprous skeletons f rom the Medieval Hospital of St. James and St. Mary Magdalene, Chichester, England
ABSTRACT
The extent and location of an inflammatory bone lesion, periostitis, were examined in 50 leprous skeletons f rom the Chichester cemetery of the Hospital of St. James and St. Mary Magdalene in Sussex, England. Although the presence of periostitis is not pathognomonic of leprosy, it predominantly indicates dermal and neuropathic changes that the patient would have presented in life. The spread of inflammation across the knee joint and the ossification of the interosseous membrane due to inflammation are also suggested.RÉSUMÉ
L'étendue et la localisation d'une lésion osseuse inflammatoire, la periostite, ont été examinés sur 50 squelettes de malades de la lèpre provenant du cimetière de Chichester de l'Hôpital de Saint Jacques et Sainte Marie-Madeleine dans le Sussex, en Angleterre. Bien que la présence d'une periostite ne soit pas pathognomonique de la lèpre, elle indique de manière prédominante des altérations dermiques et neuropathiques que le patient aurait présenté au cours de sa vie. Il y a également des indices de la propagation de l'inflammation au travers de l'articulation du genou et l'ossification de la membrane interosseuse suite à l'inflammation.RESUMEN
Se examinó el grado y la localización de lesiones óseas inflamatorias, periostitis, en 50 esqueletos de personas con lepra del cementerio Chichester del Hospital de St. James y St. Mary Magdalene, en Sussex, Inglaterra. Aunque la presencia de periostitis no es patognomónica de la lepra, indica predominantemente los cambios dérmicos y neuropáticos que el paciente debió haber tenido en vida. También se sugirió la probable diseminación de la inflamación a través de la articulación de la rodilla y la osificación de la membrana interósea debido a la inflamación.The term "periostitis" refers to the deposition of a new bone layer onto the cortical surface of the bone, under an inflamed periosteum. The periosteum is a fibrous sheath that surrounds all the bones of the skeleton, with the exception of the endocranial surface of the cranium and articular surfaces of the joints. The sheath consists of two layers; the outer layer is white fibrous tissue with a few fat cells, the inner layer consists of a dense network of fine clastic fibers (23). The inner layer of the periosteum retains its osteoblastic capacity throughout life.
The invasion of foreign organisms into any tissue of the body may cause inflammation. The periosteum can become inflamed as a result of the direct extension of a nearby infection in the soft tissue, or as a result of hematogenous spread of bacilli from a distant site. In addition, specifically in lepromatous leprosy, periosteal pain associated with an inflammatory response may occur in a type 2 reaction. This reaction, which represents an immune complex syndrome, is associated mainly with tibial periostitis (10). The resulting inflammation of the periosteum stimulates the osteoblasts of the inner periosteal layer, which lay down new deposits on the extracortical surface of the subjacent bone (Fig. 1) (17). A recurring infection results in profuse sequential layers of new bone being deposited on the cortex. Initially, the bone deposited is disorganized and has a porous appearance, it is referred to as "woven" bone and represents an active phase of infection. Later, the new bone layer becomes remodeled and organized with a system of Haversian canals; this smooth "lamellar" bone often is striated and is continuous with the original cortex of the bone. The presence of lamellar bone is diagnostic of an infection which occurred and healed well before the person's death; whereas a mix of woven and lamellar bone is considered indicative of a chronic, active infection.
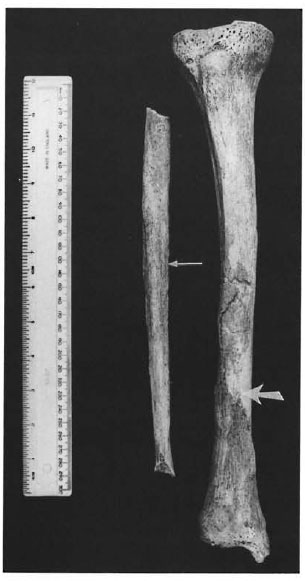
Fig. 1. Right tibia and fibula showing profuse layers of woven new bone on the subperiosteal surfaces of the shafts.
These inflammatory lesions are recognized in paleopathology but, clinically, periostitis often is not diagnosed. Although it represents a period of infection at some time during the individual's life, a thin layer of new bone can go unnoticed in clinical radiographs and may only be commented on during surgery. The aim of this paper is to discuss the prevalence of periostitis in the human skeletal remains from a specific cemetery site. Evidence from medieval leprous skeletons suggests that periostitis is manifest relatively early in the pathogenesis of leprous bone change, and is probably common in modern clinical cases where, cither through ignorance or fear, ulcerating lesions of the feet are at first neglected by the patient. In palcopathological contexts, it is hoped to illustrate how the extent and location of periostitis throughout the skeleton can provide detailed information about the clinical manifestations of leprosy in an individual.
MATERIALS AND METHODS
The remains of 355 individuals were recovered from excavations at the medieval cemetery of the Hospital of St. James and St. Mary Magdalene in Chichester, Sussex, England, between 1987-1988 (13). The hospital was founded before 1118 A. D. to house eight brethren suffering from leprosy; it was known to have functioned as a leprosarium until 1540 when it became an almshouse providing shelter for the sick and the poor.
The hospital declined around 1685-1689 and was reputed to house only one resident; the building was finally destroyed by fire in 1781 (13). The demography of the cemetery reflects the hospital's function; the western end of the cemetery was utilized in the 12th century and on excavation was found to contain a majority of male leprous skeletons. As the cemetery developed to the east it included a more equal distribution of males, females and subadults, dating from the 16th to the 17th century.
The skeletons were positively diagnosed as leprous when they displayed the-characteristic patterns of inflammation and deformation on the posteranial skeleton and features in the rhinomaxilla described by Moller-Christensen in 1953 (M). Those features are absorption of the anterior nasal spine, absorption of the alveolar process of the maxilla, inflammatory pitting and possible perforation of the oral and nasal surfaces of the palatine process of the maxilla and, in addition, the smooth absorption of the inferior zones of the margins of the nasal aperture (4). Palmar grooves on the proximal phalanges of the hands and dorsal tarsal exostoses indicated the presence of clawhand deformity and tarsal collapse (2,3,5). Septic arthritis, ankylosis, osteomyelitis, and periostitis of the tibia and fibula were accepted as denoting ulcerating lesions on the limb extremities allowing the invasion of pyogenic bacilli and their spread to deep tissues.
Some skeletons had to be omitted from the sample due to poor preservation which, among other factors, involved the crushing of the rhinomaxilla and loss of the small bones of the hands and feet during excavation. The extent and location of periostitis on the nonleprous skeletons from Chichester were recorded and compared with the prevalence of periostitis in the leprous skeletons. Tables 1 and 2 show the location of periostitis in these individuals divided into age and sex categories. The section entitled "adults" was added and incorporated into the final values since, although poor preservation prevented an estimation of age or sex in these cases, the fact that they displayed pcriostitic lesions was relevant. In the leprous sample there was a predominance of males (35) as opposed to 7 females; such a pattern is to be expected due to the nature of the leprosarium which only allowed the admittance of brethren. The occasional female skeleton may have entered the sample as a relative of the benefactors, wife of an inmate, or at a later date when the cemetery was used by the almshouse.
RESULTS
Tables 1 and 2 illustrate the percentage prevalence of the location of periostitis on the long bones of the leprous and nonleprous skeletons. Fourteen percent (50) of the 355 skeletons in the Chichester sample were diagnosed as having leprosy, and periostitis was observed on the preserved long bones of 76% (38) of these skeletons; 19% (60) of lesions followed by the fibula with 61% (37) the 305 nonleprous skeletons in the Chichand 35% (21) of the bones being affected, ester sample had evidence of periostitis on respectively. In the leprous sample, 50% (25) the long bones. In the nonleprous group the of the fibulae and 76% (38) of the tibiae had tibia was the most common site for these these lesions. The involvement of the tibiae and fibulae in leprosy may be secondary to ascending infection by pyogenic bacteria along the muscle planes of the lower limb, from ulcerated and infected feet. The small bones of the hands and feet are more commonly affected in leprosy, and it is suspected that this pattern is due to the location of ulcerated lesions on the overlying soft tissues as a result of the disease process. Involvement of the radius may be associated with an ascending infection from the hands. The nonleprous sample represents a period when the cemetery was in use as an almshouse (1540- 1600s A.D.), and it is possible that some skeletons included in this sample are of people who were suffering from leprosy but who died early in the course of the disease before pathognomonic bone involvement occurred. Some of these individuals were suffering from tuberculosis which also can result in subperiosteal new bone being deposited on the skeleton. Therefore, this group does not represent a normal distribution of periostitis in an average population. The skeletal sample originated from a hospital cemetery site and these people may have been otherwise immunocompromised either through illness or malnutrition. Some individuals in the leprous sample may display periostitis on the tibiae and fibulae as the result of local trauma sustained before they contracted leprosy. Therefore, these lesions would not be associated with the disease process and, in such a situation, it would be impossible to distinguish whether the lesions occurred before leprosy was contracted or as a result of the infection.
The extent and exact location of periostitis on 148 tibiae and fibulae also were recorded and the results are presented in Figure 2. On the fibulae, 22% (14) of the inflammatory lesions were located on the medial aspect of the shaft and 56% (36) had a diffuse covering. On the tibiae 45% (29) had periostitis on the lateral aspect of the bone and 36% (23) had a diffuse covering. The lateral border of the tibia and medial border of the fibula provide attachment for the crural interosseous membrane, the flexor and extensor digital muscles, and muscles inserted onto the tarsus. The high prevalence of periostitis on these aspects of the fibulae and tibiae suggests a spread of pyogenic bacteria from infections of the deep tissues of the plantar surfaces of the feet along the muscle planes to the lower legs. The interosseous membrane connects the borders of the tibia and fibula and intervenes between the muscles at the front and back of the leg. The fibers are largely oblique and descend laterally, although a few fibers travel medially, such as the bundle that forms the upper branch between the opening for the anterior tibial artery (21). The membrane is continuous with the interosseous ligament of the inferior tibio-fibular joint, as well as the periosteum that covers the bones. In light of this close relationship between the interosseous membrane and the muscles inserting on the bones of the foot, it is not surprising that an infected lesion on the plantar aspect of the big toe would lead to a spread of infection, initially along the muscle planes to the interosseous membrane and the periosteum. This infection may not always involve the tarsus and phalanges of the foot but may be of the soft tissue only.
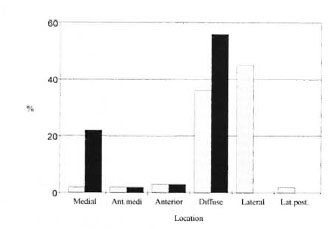
Fig. 2. Location of periostitis on tibiae/fibulae of the leprous skeletons.  = tibiae;
= tibiae;  = fibulae; Ant. medi = anterio-medial surface; Lat.post. = lateral posterior surface.
= fibulae; Ant. medi = anterio-medial surface; Lat.post. = lateral posterior surface.
DISCUSSION
Periostitis represents a nonspecific infection where the causative organisms are unknown. For example, trauma can cause inflammation of the periosteum, and the most common site of nonspecific periostitis is of the medial surface of the tibia. This area is closest to the skin surface and vulnerable to trauma. Proliferative new bone may be deposited also as part of a known disease process, for example, in venereal syphilis and leprosy where inflammation of the soft tissue and periosteum is present. An infection of the periosteum can extend to the cortex itself, resulting in osteitis, or foreign organisms may progress into the medullary cavity through hematogenous spread. If this occurs, there is subsequent suppuration and bone proliferation and destruction characteristic of osteomyelitis.
Previous discussions of periostitis in leprosy by clinicians and palcopathologists have concentrated on deposits of bone along the tibia and fibula (1,12-l4). Although these appear to be the most common sites of periosteal inflammation, they are by no means the only locations where periostitis can be found. For example, periostitis was located on the radius and ulna of Chichester skeleton 88 and probably represents a spread of infection from ulcerations on the palm of the hand (Fig. 3). Periostitis was first mentioned in connection with leprosy in 1900 when de la Camp (8) described changes in the tibiae and phalanges of the foot where the bones appeared structureless and the periosteum thickened. De la Camp considered these lesions to be the direct result of the leprosy bacillus on the periosteum and bone marrow. Periostitis was not mentioned again until 1931 when Chamberlain, et al. (7) noted periosteal thickening at the ankle and wrist joints in two of their clinical cases of leprosy.
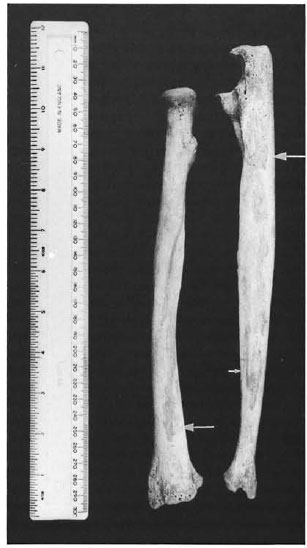
Fig. 3. Right radius and uma of Chichester skeleton 88 showing subperiosteal new bone along the borders for the attachment of the antebrachial interosseous membrane.
In 1953 Moller-Christcnscn published his results on the leprous changes in skeletons excavated from the medieval cemetery of Naestved in Denmark. He was the first to describe these lesions in relation to paleopathology. He noted a periosteal thickening on the tibiae and fibulae near the ankle joint in 91 (73.8%) of his skeletons from Naestved (15), but he considered these lesions to be undiagnostic of leprosy and difficult to distinguish from bone deposits characteristic of syphilis. Møller-Christensen also noted the occurrence of subperiosteal thickening around transverse vessels on the tibiae in 44 of his 49 cases (14). These transverse impressions were later discussed by Wells (22) and Andersen (1) and were described as vascular impressions in a "pillow" of lamellar bone. These lesions also were noticed in the Chichester sample in groups of two or three lying obliquely across the medial surface of the tibiae. However, they do not appear to follow the course of any particular vessels and are not believed to be solely vascular in origin. Their etiology is still unknown.
In 1961 Lechat (12), examining patients from Yonda in the Congo, claimed that periostitis was observed in patients with lepromatous leprosy, even in the absence of ulceration of the feet. However, it was also found in patients with all types of leprosy where plantar ulceration had occurred. Those lesions not accompanying ulceration may have been a direct result of trauma to the periosteum. Loss of proprioception would make such trauma, common in normal individuals, more likely in the leprous. Despite the brief comments by these authors, periostitis is still considered a rare phenomenon in the pathogenesis of leprosy. For example, Jopling (9) claims that ". . . despite a rare development of periostitis on the bones of the forearm and lower leg, bone damage is confined to the hands, feet and skull." Resnick and Niwayama (18) also state that "Periostitis and reactive sclerosis are usually not prominent in this disease."
However, among the 50 skeletons diagnosed with leprosy in the Chichester sample, 76% (38) had evidence of periostitis on the tibiae and fibulae. As stated earlier, Moller-Christensen was cautious about referring to periostitis on the tibia and fibula as directly related to leprosy due to the possibility of the occurrence of the trcponcmatoscs in the cemetery. However, only one skeleton, Chichester 277, shows any lesions indicative of a treponemal infection; this skeleton lies in the easterly end of the cemetery and may even represent a later inclusion since it rests on the edge of the cemetery boundaries. The majority of the skeletons diagnosed as leprous were from the western end of the cemetery and predate Columbus (1493 A.D.). Without the characteristic "caries sicca" lesions on the cranium or gummatous periostitis, described by Rothschild and Heathcote (20), a treponemal infection is unlikely and the periostitis should be considered secondary to a mycobacterial infection.
The high prevalence of periostitis in the archaeological sample is different from the pattern seen in clinical practice for several reasons. Firstly, clinicians rely heavily on radiographic evidence for their examination and diagnosis of bone lesions, unlike the palcopathologist who has the advantage of examining dry bone, where even subtle deposits of new bone on the subperiosteal surface are easily identified. Therefore, the prevalence of periostitis in a given archaeological population will be greater than that of a modern sample. Periostitis usually is recognized only during surgery or as a cloudy margin around the bone in radiographs in living patients. The more extreme osteitis and osteomyelitis alter the surface structure of the cortex, and are readily identifiable in radiographic examinations. Secondly, paleopathologists are dealing with individuals for whom there was no treatment, and the disease was able to progress "naturally."
Therefore, more severe lesions are found and recorded in paleopathology; whereas today they may be arrested by therapeutic measures.
In leprosy, inflammatory bone reaction, in the form of pitting and new bone deposition, characteristically forms on the tibiae and fibulae and the short tubular bones of the extremities. Modern patients, at the tuberculoid pole of leprosy, usually possess nerve damage of a few or only one of the peripheral nerves and, therefore, it is suspected that new bone deposition on the tibia and fibula, subsequent to sensory nerve damage and ulceration, would occur only on one of the legs. In palcopathological contexts, the distribution of bone change is considered to be indicative of the disease status history of the individual. Unilateral change only is considered to represent tuberculoid or near tuberculoid leprosy. Bilateral change indicates that the individual manifested lepromatous or near lepromatous leprosy at some time in his or her life (19). There is no way ofestimating the status ofan individual at death from the skeletal remains alone.
Periostitis, in the distribution described above, is considered to be the result of superficial and deep pyogenic sepsis and, although its presence is not pathognomonic of leprosy, it suggests dermal and neural changes that the patient may have presented in life. The possibility that some cases may be associated with type 2 reaction, particularly if only tibiae are involved, must also be considered. However, whatever this relation, the association between periostitis and the pathological processes in leprosy is clear. The presence of periostitis, osteitis and osteomyelitis each suggest a secondary invasion of pyogenic bacteria, either hematogenous or spreading via the planes of the hand and foot muscles. Such a transmission could only occur if there were open lesions on the feet or hands. Primary periostitis may occur if there is a direct extension of Mycobacterium leprae from local leprous granulomas. Therefore, soft tissue damage, unidentifiable in skeletal remains, can be suggested. For example, the superficial "glove and stocking" anesthesia and later deep anesthesia, are caused by sensory neuropathy. The loss of sensation results in minor traumatic lesions being neglected, becoming ulcerated, and allowing pyogenic bacilli to spread into the deep tissues of the limbs where they progress along the muscle planes to the periosteum. In leprosy, periostitis of the tibia and fibula is considered to be the result of pyogenic bacteria, from ulcerated lesions on the plantar surface of the foot, to the lower leg.
Foot-drop, associated with motor neuropathy, may result in disintegration of the tarsals and an increase in the surface area of the sole that can become ulcerated (11). Carpal disintegration also has been documented in leprosy (21), and ulcerated lesions may occur on the palms and wrists of an affected individual if the lesions are neglected. However, periostitis is less common on the bones of the hands and arms because it is suggested that damage to the palms would be noticed by the individual and the hands would, therefore, be more likely to be protected from further damage and infection.
Andersen (1) reported that surgeons, working on foot-drop operations, found ulcerations of the feet accompanied by empyemas of the tendon sheaths. He suggested that ascending bacteria were responsible for the inflammation and that plantar anesthesia and subperiosteal bone deposits were related. During the operations surgeons found an irregular narrowed interosseous space, which meant that passing the tendon of the tibialis posterior muscle across the interosseous space had to be abandoned in the surgical procedure. There is reason to believe that Andersen may have come across ossification of the interosseous membrane as the result of infection. Ossified segments of the crural interosseous membrane are believed to have been found on the right tibia and fibula of Chichester skeleton 115 (Fig. 4), and radio-opaque masses have been identified between the tibia and fibula on radiographs of leprous individuals from Ethiopia (Fig. 5). Inflammation of muscle (myostitis) has been reported in cases of lepromatous leprosy creating a difficulty in walking (9). Ridley (19) encountered foamy macrophages between the fibers of superficial muscles in lepromatous leprosy, and claimed that muscle tissue appeared to be unfavorable for the multiplication of M. leprae. Bryceson and Pfaltzgraff (6) report that although the bacilli may not multiply, the bacteria seem to be protected by the muscles and survive there even after treatment has eradicated them from other sites in the body. Although it has been established that myositis can occur in leprosy, ossification of the interosseous membrane is the result of osteogenesis. Because the interosseous membrane is not invested by periosteum, osteoblasts are not associated with the membrane. Resnick and Niwayama (18) claim that foci of osteoid and new bone appear in association with proliferating myositis and ossifying fascitis in the event of trauma and infection. Therefore, in light of the clinical, radiographic and palcopathological evidence, it is suggested that the spread of pyogenic bacilli to the interosseous membrane results in inflammation and the appearance of osteoblasts that deposit osteoid with subsequent ossification of the membrane.
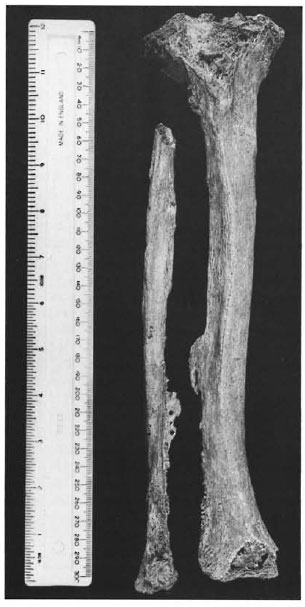
Fig. 4. Right líbia and fibula of Chichester skeleton 115 with ossification of the crural interosseous membrane at the midshaft.
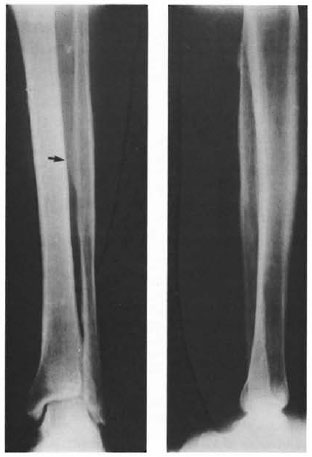
Fig. 5. Radiograph from Ethiopia showing a radio-opaque mass between the tibia and fibula of an individual with leprosy.
Acknowledgment. The authors wish to thank the Chichester District Council for providing information about the site and Jean Brown of the Department of Archaeological Sciences, University of Bradford, for producing the photographs.
REFERENCES
1. ANDERSEN, J. G. Studies in the Medieval Diagnosis of Leprosy in Denmark; An Osteoarchaeological, Historical and Clinical Study. Copenhagen: Costers Bogtrykkeri, 1969.
2. ANDERSEN, J. G. and MANCHESTER, K. The grooving of the proximal phalanx in leprosy: a palacopathological and radiological study. J. Archacol. Sci. 14(1987)77-82.
3. ANDERSEN, J. G. and MANCHESTER, K. Dorsal tarsal exostoses in leprosy: a palacopathological and radiological study. J. Archacol. Sci. 15(1988)51-56.
4. ANDERSEN, J. G. and MANCHESTER, K. The rhinomaxillary syndrome in leprosy: a clinical, radiological and palacopathological study. Int. J. Ostcoarchacol. 2(1992)121-129.
5. ANDERSEN, J. G., MANCHESTER, K. and ALI, R. S. Diaphyseal remodelling in leprosy: a palacopathological and radiological study. Int. J. Ostcoarchacol. 2(1992)211-219.
6. BRYCESON, A. and PFALTZGRAFF, R. E. Leprosy for Students of Medicine. Edinburgh: Churchill Livingstone, 1973.
7. CHAMBERLAIN, W. E., WAYSON, N. E. and GARLAND, L. H. The bone and joint changes of leprosy; a roentgenological study. Radiology 17(1931)930-939.
8. DE LA CAMP. Periostitis bei Lepra. Fortschr. Geb. Rontgcnstr. 4(1900)36-40.
9. JOPLING, W. H. Handbook of Leprosy. 3rd edn. London: William Hcincmann Medical Books Ltd., 1984, p. 28.
10. JOPLING, W. H. and MCDOUGALL, A. C. Handbook of Leprosy. 4th edn. Oxford: William Hcincmann Medical Books, 1988.
11. KULKARNI, V. N., MEHTA, J. M., SANE, S. B. and SHARANGPANI, R. C. A study of tarsal disintegration in leprosy. Proceedings of the International Conference on Biomechanics and Clinical Kinesiology of the Hand and Foot, Madras, India, 16-18 December 1985.
12. LECHAT, M.F. Mutilations in leprosy. Trop. Gcogr. Med. 13(1961)99-103.
13. LEE, F. and MAGILTON, J. The cemetery of the Hospital of St. James and St. Mary Magdalene Church: a case study. World Archacol. 21(1989)249-263.
14. MØLLER-CHRISTENSEN, V. Ten Lepers from Naestved in Denmark. Copenhagen: Danish Science Press Ltd., 1953. Med. Monogr. No. 2.
15. MØLLER-CHRISTENSEN, V. Bone Changes in Leprosy. Copenhagen: Munksgaard, 1961.
16. MØLLER-CHRISTENSEN, V. Leprosy Changes of the Skull. Copenhagen: Odcnsc University Press, 1978.
17. ORTNER, D. J. AND PUTSCHAR, W. G. J. Identification of Pathological Conditions in Human Skeletal Remains. Washington, DC: Smithsonian Institution Press, 1981. Smithsonian Contrib. Anthropol. No. 28.
18. RESNICK, R. and NIWAYAMA, G. Diagnosis of Bone and Joint Disorders. Vol. 6. 2nd edn. Philadelphia: W. B. Saunders Company, 1988, p. 2688.
19. RIDLEY, D. S. Pathogenesis of Leprosy and Other Related Diseases. Cambridge: Oxford University Press, 1988.
20. ROTHSCHILD, B. M. and HEATHCOTE, G. M. Characterisation of the skeletal manifestations of the treponemal disease yaws as a population phenomenon. Clin. Infect. Dis. 17(1933)198-203.
21. SANE, S. B., KULKARNI, V. N., SHARANGPANI, R. C. and MEHTA, J. M. A study on the disintegration of carpal bones in leprosy. Proceedings of the International Conference on Biomechanics and Clinical Kinesiology of the Hand and Foot, Madras, India, 16-18 December 1955.
22. WELLS, C. Cortical grooves in the tibia. Man 180(1963)222-223.
23. WILLIAMS, P. L. and WARWICK, R. Gray's Anatomy. 36th edn. London: Churchill Livingstone, 1984.
1. M.Sc; The Calvin Wells Laboratory, Department of Archacological Sciences, University of Bradford, Bradford BD7 1DP, U.K.
2. Ph.D; The Calvin Wells Laboratory, Department of Archacological Sciences, University of Bradford, Bradford BD7 1DP, U.K.
3. Ph.D; The Calvin Wells Laboratory, Department of Archacological Sciences, University of Bradford, Bradford BD7 1DP, U.K.
Reprint requests to Dr. Roberts.
Received for publication on 29 July 1994.
Accepted for publication in revised form on 4 November 1994.