- Volume 63 , Number 1
- Page: 92–4
"Flu" syndrome due to Rifampin; experience with four cases
In an effort to increase the utility of the JOURNAL in continuing medical education, in this section we welcome contributions dealing with practical problems in leprosy work. Submissions to this section will undergo minimal editorial changes and may well contain controversial points. Letters to the Editor pointing out other viewpoints are welcome.
Rifampin, a semisynthetic derivative of rifamycin-B, is a highly potent bactericidal drug against Mycobacterium tuberculosis and M. leprae. Of its various adverse effects, a relatively uncommon "flu" syndrome has been observed in patients with tuberculosis1-2 and leprosy.1-4 The syndrome mostly occurs with the oncc-wcckly or twice-weekly administration of rifampin and is rare with once-monthly treatment.5 However, cases are on record for whom the "flu" syndrome occurred with a regimen containing once-a-month rifampin.3-4-6-8 The syndrome appears to be due to a hypersensitivity reaction to rifampin, and antibodies against it have been demonstrated.9-10 We report herein four cases with the "flu" syndrome due to rifampin recently seen by us.
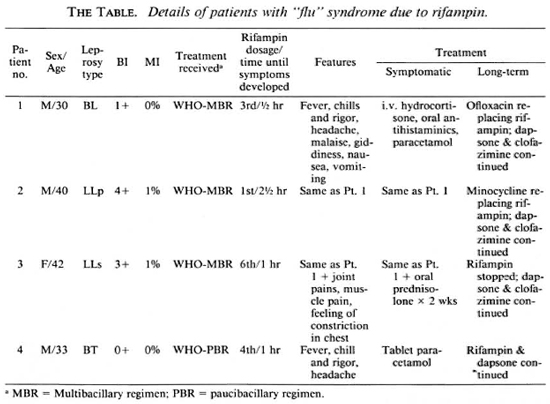
Patients were a 30-year-old male (Case 1) with borderline lepromatous (BL) leprosy, a 40-ycar-old male (Case 2) with polar lepromatous (LLp) leprosy, a 42-year-old female (Case 3) with subpolar lepromatous (LLs) leprosy, and a 33-year-old male (case 4) with borderline tuberculoid (BT) leprosy. Their bacterial and morphological indices (BI and MI) were 1 + 0%, 4 + 1%, 3 + 1% and 0 + 0%, respectively. Histopathology was consistent with the clinical diagnosis in each case. The first three patients were treated with the World Health Organization (WHO) multibacillary (MB) regimen consisting of 600 mg of monthly supervised rifampin, 100 mg daily of dapsone self-administered, 50 mg of clofazimine daily selfadministered and 300 mg monthly supervised. The fourth patient was treated with the WHO paucibacillary (PB) regimen of 600 mg monthly of rifampin supervised and 100 mg of dapsone daily self-administered. Adverse symptoms appeared half an hour after the third dose in Case 1, 2 ½ hours after the first dose in Case 2, 1 hour after the sixth dose in Case 3, and 1 hour after the fourth dose in Case 4. The first three patients developed fever with chills and rigors, headache, malaise, giddiness, nausea and vomiting. The third patient developed, in addition, joint and muscle pains and a feeling of constriction in the chest. The fourth patient had only fever with chills, rigors and headache. In Case 2 a history of taking drugs suggestive of rifampin in the past was available, but there was no history suggestive of a reaction. Case 4 could recollect a similar episode of fever and headache developing after taking antileprosy (rifampin + dapsone) treatment in the past, and the symptoms were diagnosed to be due to influenza and treated accordingly.
In all four cases, general and systemic examinations revealed a febrile state (99º-101ºF), increased pulse (80-100/min) and respiration (26-40/min) rates. The first three patients were admitted to the ward and were treated with intravenous (i.v.) hydrocortisone, oral antihistaminics and paracetamol, according to the severity of the symptoms. Paracetamol was sufficient to control the fever and arthralgia in Case 4. All vital signs, e.g., pulse, respiration, blood pressure and temperature, returned to normal within 30- 45 min after treatment in Cases 1, 2 and 4. In view of the presence of icterus in Case 3, a diagnosis of "flu"-like syndrome with hemolytic jaundice due to rifampin was made. Investigations showed hemoglobin, 10 g%; reticulocyte count, 6%; and a peripheral blood smear showed many fragmented red blood cells. An eosinophil count was 5%; serum total bilirubin, 4 mg/dl (unconjugated 3 mg/dl, conjugated 1 mg/dl); blood urea, 13 mg/dl; creatinine, 1 mg/dl; SGOT, 29U/ L; SGPT, 36U/L; alkaline phosphatase, 14 KAU. Serum for hepatitis-B surface antigen (HBS Ag) was negative. After initial treatment with intravenous hydrocortisone. Case 3 was continued on prednisolone 40 mg daily. After a week all parameters of hemolysis returned to normal and the prednisolone dose was tapered and finally stopped in 2 weeks (The Table).
Rifampin was substituted by ofloxacin (400 mg daily) in Case 1, minocycline (100 mg daily) in Case 2, and clofazimine and dapsone were continued. In Case 3 rifampin was stopped and clofazimine and dapsone were continued. In Case 4, considering the milder nature of the symptoms, rifampin was continued along with dapsone for the remaining two doses (completing a total 6-month treatment). A minor recurrence of similar symptoms after each dose could be controlled easily with paracetamol. In the remaining three cases subsequent follow up was uneventful.
DISCUSSION
Following the introduction of intermittent rifampin initially for tuberculosis, a new syndrome consisting of fever, malaise, headache, nausea, vomiting, muscle pain and arthralgia was encountered and termed a "systemic reaction."2,11 It was thought to be due to hypersensitivity to rifampin, and the demonstration of antibodies against rifampin further substantiated this hypothesis.2,10 Subsequently, such syndromes were seen to occur with once-a-month rifampin administration in leprosy as recommended by the WHO (1980) MDT regimen.3,4,7 Initially, it was believed that once-monthly dosage of rifampin is free from the "flu" syndrome side effect.1,12,13. Fortunately, such side effects are quite rare. Of 1100 patients treated with WHO regimens for MB and PB leprosy (478 WHO MBR; 522 WHO PBR), only 4 have developed the "flu" syndrome, a frequency of 0.36% (personal observations).
Various other syndromes encountered with intermittent rifampin therapy are abdominal syndrome, respiratory syndrome, cutaneous syndrome and hemolytic syndrome.5- 14 All of these symptoms probably are due to hypersensitivity to the drug and could be associated with the "flu" syndrome. In Case 3, the "flu" syndrome was associated with hemolytic jaundice, indicating a severe reaction. The other two patients had moderate symptoms. This variation in severity of the "flu" syndrome was observed by Thomas, et al. 14 Therefore, the "flu" syndrome, if mild, can be controlled with analgesics, antihistaminics and antipyretics, and does not necessitate stopping rifampin. In our Case 4 rifampin was continued because of the mild nature of the symptoms. However, moderate-to-severe "flu" syndrome warrants the immediate stopping of rifampin.
The dose of rifampin producing the "flu" syndrome was thought to be quite high (600-1200 mg),11 and a dose of 450-600 mg initially was considered to be safe. All of our patients were receiving 450-600 mg of rifampin, and the cases reported earlier also had been receiving a similar dosage.8-14 Salafia and Candida8 consider toxic psychosis to be probably another serious manifestation of the "flu" syndrome that occurs with a higher dosage of rifampin.
Our present communication highlights the fact that all medical and paramedical workers dealing with leprosy should be aware that the "flu" syndrome, although rare, is one of the side effects of rifampin. This would help them to identify this problem early, thus enabling them to manage these cases quickly to avoid misdiagnosis and more serious complications. Its early identification and management also is essential to keep up the patient's faith in the treating physician and the antileprosy drugs.
- Sandipan Dhar, M.D., D.N.B.
Senior Resident
- Inderjeet Kaur, M.D.
Associate Professor
- Vinod K. Sharma, M.D.
Associate Professor
- Bhushan Kumar, M.D., M.N.A.M.S.
Additional Professor
Department of Dermatology, Venereology and Leprology
Postgraduate Institute of Medical Education & Research
Chandigarh 160012, India
1. Girdhar, B. K. and Desikan, K. V. Pulsed rifampicin therapy in leprosy; a clinical study. Lepr. India 51(1979)475-480.
2. Poole, G., Stradling, B. and Worlledge, S. Potentially serious side effects of high-dose twice-weekly rifampicin. Br. Med. J. 3(1971)343-347.
3. Naafs, B. and Matemera, B. O. A possible "flu" syndrome on once-monthly rifampicin. Lepr. Rev. 57(1986)271-272.
4. Vaz, N., Jacob, A. J. W. and Rajendran, A. "Flu" syndrome on once monthly rifampicin: a case report. Lepr. Rev. 60(1989)300-302.
5. Jopling, W. H. and McDougall, A. C. Handbook of Leprosy. 4th edn. Oxford: Heinemann Professional Publishing Co., 1988, pp. 108-110.
6. Parking, A. A. and Shah, B. H. "Flu" like syndrome with rifampicin pulse therapy. Indian J. Lepr. 61(1987)209-210.
7. Patki, A. H., Jadhav, V. H. and Mehta, J. M. "Flu" syndrome on once monthly rifampicin. Indian J. Lepr. 60(1988)84-86.
8. Salafia, A. and Candida. Rifampicin induced "flu" syndrome and toxic psychosis. Indian J. Lepr. 64(1992)537-539.
9. Proceedings of Workshop on Intermittent Drug Therapy and Immunological Implications in Anti-TB Treatment with Rifampicin. Scand J. Respir. Dis. Suppl. 84(1973).
10. Riska, N. and Madson, K. Adverse reactions during rifampicin treatment. Scand. J. Respir. Dis. 53(1972)87-96.
11. Girling, D. J. and Hitze. K. L. Adverse reactions to rifampicin. Bull. WHO 57(1979)45-49.
12. Girling, D. J. and Fox, W. Side effects of intermittent rifampicin. Br. Med. J. 4(1971)231-232.
13. World Health Organization. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.
14. Thomas, A., Joseph, P. and Prabhakar, R. "Flu" syndrome associated with other systemic manifestations with once a month rifampicin in the treatment of multibacillary leprosy. Indian J. Lepr. 65(1993)219-224.
Reprint requests to Dr. Kumar.