- Volume 63 , Number 1
- Page: 95–6
Changes in leprosy clinical pattern after multidrug therapy implementation
To the Editor:
This department is for the publication of informal communications that are of interest because they are informative and stimulating, and for the discussion of controversial matters. The mandate of this JOURNAL is to disseminate information relating to leprosy in particular and also other mycobacterial diseases. Dissident comment or interpretation on published research is of course valid, but personality attacks on individuals would seem unnecessary. Political comments, valid or not, also are unwelcome. They might result in interference with the distribution of the JOURNAL and thus interfere with its prime purpose.
Although the introduction of the multidrug therapy (MDT) regimen for leprosy treatment has been followed by a consistent reduction in prevalence rates in many countries all over the world, its effect on transmission, measured by the incidence rates, can be seen only in a long-term evaluation. The immediate effect of the shortening of the length of treatment is to reduce the duration of the disease (2,10,13). The influence of MDT on the frequency of different types of leprosy, whether multibacillary or paucibacillary leprosy rates, has not been described as yet for the Brazilian surveillance system.
In Brazil, MDT was only introduced in 1986. In contrast with the worldwide trend, detection rates have increased over the last three decades (9). Around 180,000 leprosy patients are registered and under treatment with more than 33,000 newly diagnosed cases in 1993, with prevalence rates of 12.51/ 10,000 inhabitants and detection rates of 22.29/100,000 inhabitants (Brazilian Ministry of Health. Programa nacional de eliminação, dados epidemiológicos e operacionais. Coordenação Nacional de Dermatologia Sanitaria. Informes Técnicos 1986/ 1994, Brasilia).
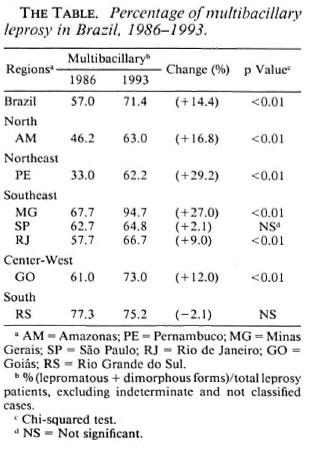
The frequency of different types of newly diagnosed leprosy cases registered before (1986) and after (1993) MDT implementation were compared. Data from the regular surveillance system on multibacillary (MB; lepromatous and dimorphous clinical forms) and paucibacillary (PB; tuberculoid) cases from representative Brazilian states of high (Amazonas/AM, Goias/GO, Pernambuco/PE, Minas Gerais/MG, Rio de Janeiro/RJ); intermediate (Sao Paulo/SP) and low endemicity (Rio Grande do Sul/RS) were collected to describe the clinical epidemiological pattern ofleprosy. These comprised a total of 11, 542 newly detected cases in a population of nearly 87,000,000 inhabitants. Indeterminate (I) clinical forms of leprosy were not included, considering the controversy on the classification and treatment of early lesions (6-11). To have a more consistent epidemiological profile the data were analyzed by each state since this is the program management unit.
The detection rate for MB leprosy increased sharply from 5.6 to 11.7/100,000 in the period 1986-1993. A stable rate of approximately 5/100,000 was observed for PB cases (The Figure). This selective change was seen in nearly all Brazilian states with a increasing trend to allocate patients to a 24-month regimen (MB) as opposed to the 6-month (PB) treatment regimen (The Table).
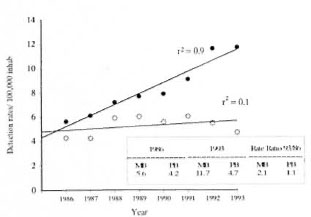
The figure. Detection rates of MB and PB leprosy in Brazil, 1986-1993. ● = MB; ○ = PB; r2 = correlation coefficient.
In an equivalent period of time (1969- 1987) before the introduction of MDT, the overall detection rates in Brazil rose from 6 to 17/100,000. An annual increase of tuberculoid cases was higher (5%) than lepromatous/borderline forms (3%) according to the epidemiological data of the Brazilian Health Ministry. Cases also were detected at a younger age, suggesting that there was a real increase in the transmission of the disease (5-9).
The increase in detection rates reported here may represent, to some extent, an improvement of the surveillance system on the availability and quality of health services and/or urbanization of leprosy. The implementation of MDT has caused an improvement in the quality of leprosy routine assistance and in coverage. In addition, the urbanization of tropical diseases has been well documented and leprosy is no exception (8).
The rapid increase in MB leprosy within less than a decade deserves careful examination. This pattern is not consistent with a situation of high endemicity in which tuberculoid forms are predominant and cases are detected at a younger age. Moreover, changes in leprosy type patterns seem unusual in a short time interval (3). The introduction of MDT was the only major modification on a nationwide scale, since no significant change in cither the surveillance system or leprosy case definition/classification (Madrid system) has occurred. This change in the treatment routine appeared to have a powerful impact on the classification of the newly detected leprosy cases. The shift in leprosy frequency toward MB after the introduction of MDT seems to reflect a conservative behavior in the clinical decision when allocating patients to treatment, rather than a real change in the epidemiologic profile. It suggests that MDT implementation may be leading to a misclassification bias by overestimating the frequency of MB forms, possibly due to an excess of dimorphous forms. As previously reported, most MB cases are, in fact, smearnegative patients (7). Unfortunately, the available data do not allow one to discriminate between lepromatous and dimorphous types since they are considered together in the reports, and there are no published data on the percentage of smear positivity in each of these categories.
The over-allocation of the patients as MB seems to be an operational problem. Clinicians tend not to rely on skin-smear results and do prefer to choose longer treatment for leprosy patients. In addition, the Brazilian Leprosy Control Programme had emphasized and even recommended the allocation of doubtful patients to the MB regimen to ensure that no patient receives insufficient treatment. The possible effects of MDT therapy on the epidemiological trends and on the classification of leprosy have been reported recently (I,4,12).
Although MDT has been introduced only lately in Brazil, almost 100% coverage has been reached in the last 2 years. This points out the acceptability of the MDT regimens by both providers and patients. For optimal allocation of patients to treatment, diagnosis accuracy and detection of operational problems in classifying cases is urged. The critical question is how much MB overdiagnosis is acceptable for treatment assignment purposes and to provide epidemiological indicators for allowing type-specific trend analysis?
The Brazilian Leprosy Control Programme already has recognized the bias reported in the allocation of patients and has been recommending the use of bacilloscopy, whenever possible, to assign patients to MB or PB groups. Indeterminate cases previously allocated as MB are considered PB regardless of the Mitsuda test.
A health system and epidemiology research are required in order to understand regional differences and improve diagnosis accuracy, compliance and coverage. These are essential tools to achieve community effectiveness in any drug regimen control strategy.
- Celina M. T. Martelli, Ms.C.
Ana Lucia S. S. Andrade, Ms.C.
Departamento de Saude Coletiva
Instituto de Patología Tropical e Saude Publica
Universidade Federal de Goias, Brasil
- Maria Aparecida F. Grossi, Ms.C.
Maria Ana A. Leboeuf, M.D.
Secretaria Estadual de Saude
Minas Gerais, Brasil
-Clovis Lombardi, Ph.D.
Fabio Zicker, Ph.D.
Organización Panamericana de la Salud, Venezuela
REFERENCES
1. CARTEL, J.L., SPIEGEL, A., NGOC, L.N., MOULIA-PELAT, J.P., MARTIN, P.M.V. and GROSSET, J.H. Leprosy in French Polynesia; the possible impact of multidrug therapy on epidemiological trends. Lepr. Rev. 63(1992)223-230.
2. FINE, P.E.M. Reflections on the elimination of leprosy. (Editorial) Int. J. Lepr. 60(1992)71-80.
3. IRGENS, L.M . and SKJAEVEN, R. Secular trends in age at onset, sex ratio, and type index in leprosy observed during declining incidence rates. Am. J. Epidemiol. 122(1985)695-705.
4. JESUDASAN, K., VIJAKUMARAN, P., PANNIKAR, V.K.. and CHRISTIAN, M. Impact on leprosy as measured by selective indicators. Lepr. Rev. 59(1988)215-223.
5. LOMBARDI, C. Tendencia secular da detecção da hanseniase no estado de São Paulo. Rev. Pat. Trop. 22(1993)407-487.
6. LOMBARDI, C, COHEN, S., LEIKER, D.L., SOUZA, J.M.P., CUNHA, P.R., MARTELLI, C.M.T., ANDRADE, A.L.S.S. and ZICKER. F. Agreement between histopathological results in clinically diagnosed cases of indeterminate leprosy in São Paulo, Brazil. Acta Leprol. (1994). (in press)
7. MARTELLI, C.M.T., ANDRADE, A.L.S.S., SILVA, I.M., SOUZA. L., COSTA, M.B., MORAES NETO, O.L. and ZICKER, F. Consideraçaaes sobre classificação e definição de caso em hanseniase nos programas de controle. Rev. Soc. Bras. Med. Trop. 27 Suppl. 1(1994)21.
8. MARTELLI, C.M.T., MORAES NETO, O.L., ANDRADE, A.L.S.S., SILVA, S.A., SILVA, L.M. and ZICKER, F. Spatial patterns of Leprosy in an urban area in central Brazil. Bull. WHO (1994). (in press)
9. MOTTA, C. P. and ZUNIGA, M.G. Time trends of hansen's disease in Brazil. Int. J. Lepr. 58(1990)453-461.
10. NORDEEN, S.K. Leprosy 1962-992; Epidemiology and control of leprosy-a review of progress over last 30 years. Trans. R. Soc. Trop. Med. Hyg. 87(1993)515-517.
11. PANNIKAR, V.K . Defining a case of leprosy. Lepr. Rev. 63 Suppl.(1992)61s-65s.
12. PATTYN, S. R. Future trends in the treatment of leprosy. Trop. Geogr. Med. 462(1994)85-88.
13. SMITH, P.G. Revised estimates of global leprosy numbers. Lepr. Rev. 62(1992)317-318.
Reprint requests to Dr. Celina Maria Turchi Martelli, Rúa 132-A No. 74, Setor Sul, 74093-220 Goiania Goias, Brasil.