- Volume 63 , Number 1
- Page: 113–5
Successful treatment of a lepromatous patient with clarithromycin
To the Editor:
In 1992, we initiated a clinical trial in lepromatous leprosy of clarithromycin designed to evaluate clinical response and the killing of Mycobacterium leprae by an initial 1-g dose followed subsequently by 1 g daily for 3 months. For these purposes we had planned to recruit eight previously untreated BL/LL patients but, unfortunately, after the completion of but one trial patient the San Francisco laboratory was closed; since there are only a few published pilot trials (1,4) of the use of clarithromycin in leprosy patients and none by these same methods, we report the results from our sole patient.
The patient, a 24-year-old Filipino male, had a family history of leprosy and had noted a generalized nodular eruption for the preceding year. No nerve enlargement or deformation were noted and Semmes-Weinstein monofilament testing was within normal limits. An ophthalmologic exam revealed bilateral keratitis and beaded corneal nerves confined to the left eye. Slit-skin smears from six sites yielded an average bacterial index (BI) of 3.7. Skin biopsy was reported by the Ridley-Jopling classification as "polar LL."
Initially, the patient underwent clinical evaluation, had a routine hemogram, and blood chemistries were performed. After informed consent was obtained, the patient underwent a skin biopsy and took a single 1-g dose of clarithromycin. The viability of M. leprae in the pretreatment biopsy and its sensitivity to dapsone (0.0001%, 0.001%, and 0.01%) and clarithromycin (0.001%, 0.01 %, and 0.1 %) were determined. Following a skin biopsy performed 1 week after this single initial dose of clarithromycin, the patient was treated with clarithromycin 1 g daily for the next 3 months. Clinical evaluation and skin biopsies for M. leprae viability were performed on all subsequent clinic visits, i.e., 2 weeks, 1 month, 2 months, and 3 months after beginning daily therapy. From the pretreatment biopsy and subsequent ones 5000 M. leprae were inoculated into both hind feet of groups of female BALB/C mice (Jackson Laboratories, Bar Harbor, Maine, U.S.A.). The viability of M. leprae from these biopsies was determined from pools of four hind foot pads (two mice) harvested 12 months subsequently, as well as at times from 10 individual foot pads. In both instances viable M. leprae were considered to be present in the initial inoculum if > 105 M. leprae /foot pad were harvested.
Four days after the initial single clarithromycin dose the patient experienced a 2-min episode of abdominal cramps which resolved spontaneously and did not recur. On clinical evaluation a week after the single clarithromycin dose there was no observed change in the nodular skin lesions.
By 2 weeks of daily therapy there was a decrease in infiltration, perhaps as much as 50% in some lesions. Over the subsequent 2 ½ months, skin infiltration continued to decrease, with leprous nodules becoming progressively softer and flatter. However, by completion of therapy the infiltrated nodules were still apparent and slightly hyperpigmented. At that time the patient was switched to our usual regimen of daily dapsone 100 mg and daily rifampin 600 mg. During the course of the trial no other side effects, no reactional states, and no laboratory abnormalities were observed. The patient's pretreatment M. leprae isolate grew in both the four mouse foot pad pools and in some mouse single feet, and was found fully sensitive to dapsone (0.0001% in the diet). This patient's M. leprae were inhibited by clarithromycin 0.01% and 0.1%, but not 0.001% in the diet. Previous studies utilizing other M. leprae isolates in mice have reported inhibition of M. leprae multiplication to require a dietary concentration of clarithromycin as low as 0.001 % and as high as 0.1% (2,3,5).
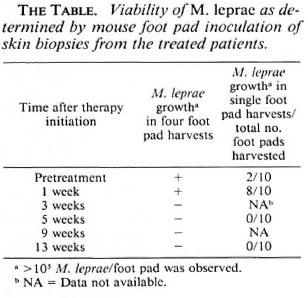
The viability of M. leprae from sequential skin biopsies is presented in The Table. Single-dose therapy had no measurable effect on M. leprae viability. However, no viable M. leprae were detected in four mouse foot pools inoculated with skin-biopsy specimens obtained 2 weeks, 1 month, 2 months, and 3 months after the initiation of daily therapy or in any of 10 single mouse foot pads infected with biopsies obtained 1 and 3 months after the inception of daily clarithromycin therapy.
Previously, Ji, et al. (4) found that clarithromycin 500 mg daily both alone (12 patients) and combined with 100 mg minocycline (11 patients) eliminated all viable M. leprae by 1 and 2 months of therapy. Chan, et al. (1) treated nine lepromatous patients with clarithromycin 1500 mg two times daily on the first day, followed by no therapy for 1 week then 1000 mg daily for 2 weeks and, finally, 500 mg daily for 6 weeks thereafter. In that study the results found were remarkably similar to those obtained in our own patient: the initial 3-g dose had no effect on M. leprae viability, but in all patients no viable bacilli were found at 3, 5, and 8 weeks after the initiation of the trial. As in our patient, in both published trials (1,4) the side effects were found to be minimal, and no laboratory abnormalities were detected.
In summary, as in two previously published pilot trials, our single clarithromycintreated patient had a good clinical response, minimal side effects, and no resultant laboratory abnormalities. Loss of M. leprae viability was, as in one of the pilot trials (1), not obtained by a single day of treatment. On the other hand, in our patient and the other two studies (1,4), by a few weeks of daily therapy and thereafter all viable M. leprae bacilli had been consistently eliminated by the means employed. Thus, as in previous trials (1,4) , clarithromycin appeared in our patient to be remarkably efficacious.
- Robert H. Gelber, M.D.
Medical Director
San Francisco Regional Hansen's Disease Program
2211 Post St., Suite 301
San Francisco, CA 94115, U.S.A.
REFERENCES
1. CHAN. G. P., GARCIA-IGNACIO, B. Y., CHAVEZ. V. E., LIVELO, J. B., JIMENEZ, C. L., PARRILLA, M. L. R. and FRANZBLAU, S. G. Clinical trial of clarithromycin for lepromatous leprosy. Antimicrob. Agents Chemother. 38(1994)515-517.
2. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
3. GELBER, R. H., MURRAY, L. P., Siu, P. and TSANG, M. Clarithromycin at very low levels and on intermittent administration inhibits the growth of M. leprae in mice. Int. J. Lepr. 60(1992)485-487.
4. Jr, B.. JAMET, P., PERANI, E. G., BOBIN, P. and GROSSET, J. H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(11993)188-190.
5. WALKER. L. L., VAN LANDINGHAM, R. M. and SHINNICK, T. M. Clarithromycin is bactericidal against strains of Mycobacterium leprae resistant and susceptible to dapsone and rifampin. Int. J. Lepr. 61(1993)59-65.