- Volume 63 , Number 1
- Page: 118–20
Patient treatment compliance in leprosy; an unjustifiably critical review
To the Editor:
My attention has been drawn to your editorial entitled "Patient Compliance in Leprosy: A Critical Review" by Vadher and Lalljee (Int. J. Lepr. 60, 1992, 587). Unfortunately, this review contained many important inaccuracies, unjustifiably criticized several of the compliance investigations undertaken by my colleagues and me, and failed to consider other highly pertinent studies that we had conducted.
Despite Vadher and Lalljee's assertion that methodological issues such as the definition and classification of compliance were rarely given due prominence in Huikeshoven's (11) and my (5) previous reviews on the subject, their review failed to cite or discuss our original paper (3) describing the basis of the dapsone/creatinine (D/C) ratio method for monitoring the self-administration of dapsone. The investigation reported in this paper demonstrated the severe limitations of trying to monitor dapsone ingestion using qualitative spot tests based on the reaction of dapsone and its metabolites with Ehrlich's reagent (p-dimethylamino-benzaldehyde) (1), primarily because of the relatively slow elimination of dapsone and its metabolites. As a consequence, the positivity of qualitative dapsone urine tests is markedly influenced by diuresis.
It was for this reason that we recommended estimating dapsone and its diazotizable metabolites by the more specific Bratton and Marshall procedure (2) and allowing for the effects of diuresis by ratioing to creatinine using the simple alkaline picrate method (4). We then described how the overall percentage of dapsone doses being taken by a group of leprosy patients could be calculated by estimating the mean test D/C ratio (T) of their urine samples and comparing it with the average supervised D/C ratio (S) of urine samples collected from a similar group of patients receiving the same daily dose of dapsone under supervision. In each case, values were corrected for the levels of normal diazotizablc compounds present in the urine by determining the mean blank D/C ratio (B) of samples from another group of subjects not ingesting dapsone [% ingested doses = 100 (T - B)/(S - B)].
Vadher and Lalljee also failed to discuss the basis for the interpretation of individual urinary D/C ratios (10) or the essential conflict between discovering tests capable of providing unambiguous estimates of the extent of patient compliance and their simplicity (5).
The use of the pharmacologically inert marker substance isoniazid in compliance studies also should have been referred to since it enables parallel independent evidence concerning the regularity of drug selfadministration to be obtained (7). We used isoniazid in this way in two of the studies summarized in Vadher and Lalljce's table (8,9), but the fact that INH stood for isoniazid and the interpretation of urine tests to detect its metabolites, isonicotinic acid and acetylisoniazid, were not explained. Two other studies (16,17) in which we used isoniazid as an innocuous marker to aid the assessment of the regularity of the self-administration of both dapsone and of other antileprosy drugs were not cited.
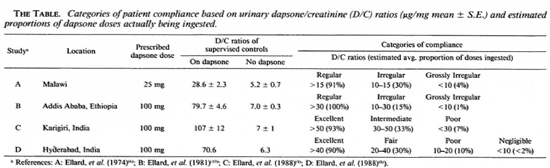
Dividing up subjects whose drug self-administration was clearly irregular into different categories is, of course, arbitrary but is valuable in that it shows the "spread" in behavior. However, as will be seen from The Table based on the results of the 4 studies carried out by me and my colleagues (6,8-10 ), which were specifically criticized by Vadher and Lalljee for using conflicting arbitrary compliance classifications, such criticism was clearly unwarranted. Unfortunately, in their editorial Vadher and Lalljee failed to appreciate the fact that when the study was undertaken in Malawi the patients were prescribed daily doses of only 25 mg, whereas in the subsequent studies carried out in Ethiopia and India 100 mg dapsone doses were given. Thus, it was entirely appropriate to use different D/C ratio definitions for the three or four compliance categories. Since these definitions were based on the D/C ratios of urine samples from groups of similar patients being treated with cither 25 mg or 100 mg supervised daily dapsone doses, they allowed both for differences in the dosages of dapsone being prescribed as well as for any potential racial or sex-related differences in the metabolism and clearance of dapsone and its metabolites, or in the excretion of creatinine. The appropriateness of these different classifications can be seen in our original papers; indeed, in three of the papers the proportions of dapsone doses being ingested by patients in the various compliance categories were set out in tabular form.
Another major criticism of our studies by Vadher and Lalljee was that our patients were selected for atypically good compliance; hence, our findings were biased. However, in most of our studies patients were not told why the urine samples used to monitor their compliance were being collected and often only a single urine sample was collected per subject.
The one study (8) that was singled out for Vadher and Lalljee's most severe criticism of potential selection bias was that in which repeated urine samples were collected both at the clinic and by means of random surprise home visits over an 18-month period. Furthermore, patients were only included in this study if they were thought likely to continue treatment for at least 2 years. No significant differences were found between the results from the clinic and home-visit samples, and the regularity of apparent drug self-administration did not change with time. The study also showed that notwithstanding the considerable patient selection, there was a continuous spectrum in the regularities of drug taking extending right down to less than 10% of the prescribed doses. In our report on the study we pointed out that many of the patients "selected" in this way, who came regularly every month to collect their medication, nevertheless ingested the drugs they had collected extremely irregularly. To have tried to assess the compliance of patients who either absconded from treatment or collected it very irregularly would not only have been virtually impossible but also irrelevant since if patients did not collect their drugs, by definition they could not ingest them.
Contrary to Vadher and Lalljee's claim, the past treatment histories (up to 20 years' monotherapy with dapsone) of the multibacillary patients whose dapsone and clofazimine compliance was studied on WHOrecommended multidrug therapy (MDT) (18) in Karigiri in the context of the WHO-supported THELEP controlled trial of its efficacy (9) were, in fact, typical of multibacillary patients the world over. There was, however, one important almost unavoidable "bias" attached to this study, namely, the exceptional standards of organization and patient care at Karigiri. Indeed, it was largely for these reasons that Karigiri was chosen as a center for one of the first two THELEP-sponsored controlled clinical trials of WHO-MDT.
In the discussion of the results of this investigation we pointed out that the results confirmed the excellent compliance of patients in Karigiri demonstrated previously when patients were being treated with dapsone monotherapy (13). Such regularity in leprosy drug self-administration was quite exceptional. In other parts of India and the rest of the world it was common to find that only about half of the prescribed dapsone treatment was actually being ingested (5). However, as we explained in the discussion section of our paper, this "bias" did not affect the main conclusions of the investigation, namely, that overall drug acceptability was excellent and that there was a marked correlation between the self-administration of daily dapsone and clofazimine so that patients at greatest risk of developing rifampin resistance because of poor dapsone compliance were the very ones most unlikely to take clofazimine daily. Furthermore, as a consequence of the "bias," we concluded that "the results obtained emphasized the importance of employing regimens containing high degrees of supervised drug administration, especially in areas where drug compliance is known to be poor."
Only later, after our paper was published, did I discover the probable reason for the exceptionally good patient compliance at Karigiri -that they had had for many years a policy of routinely testing urine samples by the D/C ratio method and informing all those patients with low ratios that the staff knew that they were not taking their treatment regularly.
In retrospect, I believe these studies that my colleagues and I undertook, as well as those of others who also used the D/C ratio method, helped to demonstrate the reality and ubiquity of irregular drug self-administration. Thus, just as in the dapsone monotherapy era, dapsone-resistant strains of Mycobacterium leprae were found wherever they were sought; so, too, was poor compliance. Both demonstrations rightly pointed to the importance and urgency of switching from dapsone monotherapy to multidrug treatment with potent intermittent regimens enabling an important part of the drug treatment to be ingested under supervision. Recent demonstrations of the potential efficacy of supervised monthly doses of clarithromycin, clofazimine, minocycline, ofloxacin, and or sparfloxacin (12,15) show that further strengthening of the already marvelously effective standard WHO-MDT regimen for multibacillary patients (14) can be anticipated.
- Gordon A. Ellard, Ph.D.
Department of Medical Microbiology
St. George's Hospital Medical School
Cranmer Terrace
London SW17 ORE. U.K.
REFERENCES
1. BALAKRISHNAN, S. A note on the screening of DDS in urine by spot test. Lepr. India 41(1969)77-78.
2. BRATTON, A. C. and MARSHALL, E. K. A new coupling component for sulfanilamide determination. J. Biol. Chem. 128(1939)537-550.
3. ELLARD, G. A. Urine tests to monitor the self administration of dapsone by leprosy patients. Am. J. Trop. Med. Hyg. 23(1974)464-470.
4. ELLARD, G. A. Profile of urinary dapsone/creatinine ratios after oral dosage with dapsone. Lepr. Rev. 51(1980)229-236.
5. ELLARD, G. A. Drug compliance in the treatment of leprosy. Lepr. Rev. 52(1981)201-213.
6. ELLARD, G. A., GAMMON, P. T. and HARRIS, J. M. The application of urine tests to monitor the regularity of dapsonc self-administration. Lepr. Rev. 45(1974)224-234.
7. ELLARD, G. A., JENNER, P. J. and DOWNS. P. A. An evaluation of the potential use of isoniazid, acctylisoniazid and isonicotinic acid for monitoring the self-administration of drugs. Br. J. Clin. Pharmacol. 10(1980)369-381.
8. ELLARD, G. A., KIRAN, K. U. and STANLEY, J. N. A. Long-term prothionamide compliance: a study carried out in India using a combined formulation prothionamide, dapsone and isoniazid. Lepr. Rev. 59(1988)163-175.
9. ELLARD, G. A., PANNIKAR, V. K., JESUDASAN, K. and CHRISTIAN, M. Clofazimine and dapsonc compliance in leprosy. Lepr. Rev. 59(1988)205-213.
10. ELLARD, G. A., PEARSON, M. H. and HAILE, G. S. The self-administration of dapsone by leprosy patients in Ethiopia. Lepr. Rev. 52(1981)237-243.
11. HUIKESHOVEN. H. Patient compliance with dapsonc administration in leprosy. (Editorial) Int. J. Lepr. 49(1981)225-258.
12. JAMET, P., TRAORE. I., HUSSER, J. A. and Jt, B. Short-term trial of clofazimine in previously untreated lepromatous leprosy. Int. J. Lepr. 60(1992)542-548.
13. JESUDASAN, K., GEORGE, C. J. G., TAYLOR, P. M., KURIAN, P. V. and JOB, C. K. An evaluation of the self-administration of DDS in Gudiyatham Taluk. Lepr. India 48 Suppl.(1976)668-676.
14. Jt, B. and GROSSET, J. H. Recent advances in the chemotherapy of leprosy. Lepr. Rev. 61(1990)313-329.
15. Jt, B., PERANI, E. G., PETINON, C. and GROSSET, J. H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
16. STANLEY, J. N. A., PEARSON, J. M. and ELLARD, G. A. An investigation of dapsone compliance using an isoniazid-marked formulation. Lepr. Rev. 51(1980)317-325.
17. STANLEY, J. N. A., PEARSON, J. M. H. and ELLARD, G. A. Ethionamide, prothionamide and thiacetazone self-administration; studies of patient compliance using isoniazid-marked formulations. Lepr. Rev. 57(1986)9-18.
18. WHO STUDY GROUP. Chemotherapy of leprosy for control programmes. Geneva: World Health Organization, 1982. Tech. Rep. Ser. 675.