- Volume 62 , Number 4
- Page: 552–8
A longitudinal study of immunologic reactivity in leprosy patients treated with immunotherapy
ABSTRACT
More than 150 leprosy patients treated with multidrug therapy (MDT) plus immunotherapy (IMT) with a mixture of heatkilled Mycobacterium leprae plus live BCG were studied in relation to humoral and cellmediated immune responses. Many previously had received prolonged sulfone monotherapy. Patients received 2 to 10 doses of IMT in a period of 1 to 3 years, depending upon their clinical form of leprosy. The patients were followed up for 5 to 10 years with repeated determinations of antibody levels to phenolic glycolipid-I; lymphopro liferative (LTT) responses to soluble extract of M. leprae, to whole bacilli and to BCG, skin-test responses and bacterial indexes (Bis). After MDT plus IMT there was a statistically significant decrease of antibody levels in the multibacillary (MB) group. The BI decreased proportionally to the ELISA results. LTT increased to M. leprae antigens, especially to soluble extract, in a high percentage of these patients (34% of LL patients positive). Lepromin positivity in MB patients increased f rom 5% initially positive to 75% at the cut-off" during this follow up. These results show substantial early and persistent cell-mediated reactivity to M. leprae in many MB patients treated with MDT-IMT, confirming and expanding previously published data.RÉSUMÉ
Plus de 150 malades de la lèpre traites par polychimiothérapic (PCT) plus immunothérapie (IMT) avec un mélange de Mycobacterium leprae tués par la chaleur et du BCG vivant ont été étudiés quant à leurs réponses immunitaires de type humoral et à médiation cellulaire. Beaucoup avaient reçus auparavant une monothérapie à la dapsone de longue durée. Les patients ont reçu 2 à 10 doses d'IMT sur une période de un à trois ans, selon le type clinique de lèpre. Ils ont été suivis pendant 5 à 10 ans avec détermination répétée des taux d'anticorps vis-à-vis du glycolipide phénolique-I, des réponses de prolifération lymphocytaire (PLT) à de l'extrait soluble de M. leprae, à des bacilles entiers et au BCG, des réponses à des tests cutanés et des indices bactériens (IB). Après PCT plus IMT, il y avait une diminution statistiquement significative des taux d'anticorps dans le groupe des multibacillaires (MB) . L'IB diminuait de manière proportionnelle aux résultats du test ELISA. La PLT augmentait vis-à-vis des antigènes de M. leprae, particulièrement vis-à-vis de l'extrait soluble, dans un grand pourcentage de ces patients (34% des patients LL positifs). La positivité à la lépromine augmentait chez les patients MB, de 5% positifs initialement à 75% durant cette période. Ces résultats montrent une réactivité à médiation cellulaire significative, précoce et persistante vis-à-vis de M. leprae chez de nombreux patients MB taités par PCT-IMT, ce qui confirme et développe des données publiées antérieurement.RESUMEN
Se estudió la respuesta inmune humoral y la respuesta inmune celular en más de 150 pacientes con lepra tratados con poliquimioterapia (PQT) más inmunoterapia (IT) con un mezcla de Mycobacterium leprae muerto por calor y BCG viable; muchos pacientes habían recibido monoterapia prolongada con sulfona. Los pacientes recibieron de 2 a 10 dosis de IT en un periodo de 1 a 3 años, dependiendo de su forma clínica de lepra. Los pacientes se valoraron durante 5 a 10 años mediante determinaciones periódicas de niveles de anticuerpos anti-glicolípido fenólico-I, reacciones de linfoproliferación en respuesta al M. leprae y al BCG, reacciones intradérmicas, e índices bacterianos (IBs). El tratamiento con PQT más IT condujo a una disminución estadísticamente significativa en los títulos de anticuerpos en el grupo multibacilar (MB). El IB disminuyó proporcionalmente a los resultados por ELISA. La linfoproliferación inducida con antígenos de M. leprae, especialmente con un extracto soluble, aumentó en un elevado porcentaje de estos pacientes (33%). La positividad a la lepromina en los pacientes multibacilares aumentó del 5% al 75%. Estos resultados, que muestran una temprana y persistente respuesta inmune celular contra el M. leprae en muchos de los pacientes MB tratados con PQT e IT, confirman y extienden las observaciones publicadas previamente.Leprosy is a chronic granulomatous disease caused by Mycobacterium leprae. The diverse clinical forms of the disease arc closely related to the degree of cell-mediated immunity of the patient. The role played by regulatory T lymphocytes has been reported, mainly of helper phenotype CD4+ cells (13). Patients with tuberculoid leprosy, who develop typical granulomatous lesions formed by well-differentiated macrophages with few intracellular bacilli and many CD4+ T cells surround by a cuff of CD8+ cells, show strong cell-mediated immunity and low levels of antibodies against phenolic glycolipid-I (PGL-I). Patients with lepromatous, borderline lepromatous and Mitsuda-negative indeterminate leprosy do not develop resistance nor cell-mediated reactions associated with delayed-type hypersensitivity. Whether this type of persistent anergy reflects a primary macrophage defect resulting in inadequate antigen presentation or other mechanisms has not been clarified. The inappropriate response results in an inadequate cell-mediated immune response characterized, together with other features, by activation of suppressor cell populations (10,14) and lack of an adequate stimulus of effector T cells. In some cases this defect can be restored in vitro by adding exogenous lymphokines (6) or by stimulation with components of fractionated mycobacteria (6,15). In multibacillary leprosy, antibody levels can be used to monitor the elimination of the bacillary load in patients submitted to various types of treatment (1,3,12).
In the search for a vaccine against leprosy, a mixture of heat-killed M. leprae and viable BCG has been evaluated in the immunoprophylaxis and immunotherapy of the disease at the Institute of Biomedicine, Caracas, Venezuela. Convit, et al. (4,5) have reported clinical and bacteriological improvement as well as the development of a positive skin-test reactivity in 60% to 70% of borderline lepromatous and lepromatous patients after 8 to 10 doses of the combined vaccine. An independent study has demonstrated histopathological changes toward more resistant forms of the disease in a high percentage of these patients (11).
In this study, we have evaluated the response of patients submitted to immunotherapy (IMT) with this combined vaccine together with multidrug therapy (MDT) during a 5- to 10-year period. Based on the use of multiple criteria, we have been able to analyze the diverse manifestations of the immune responses in these patients, most of whom had multibacillary disease. The parameters evaluated included determinations of the levels of antibodies against PGL-I, lymphocyte transformation assays, bacterial indexes and intradermal tests before each IMT treatment and at regular intervals for a period of at least 5 years.
MATERIALS AND METHODS
Patients. The patients were seen in the Clinical Section of the Institute of Biomedicine. Clinical, bacteriological and histopathological criteria were used to classify the form of disease according to the Ridley-Jopling scale (16). Twenty percent of the multibacillary patients had received sulfone therapy for more than 20 years prior to IMT and the remainder had received previous chemotherapy for an average of 6 years; all received multidrug therapy (MDT) together with IMT in this evaluation. The study included patients with active lesions and patients who were bacteriologically negative.
Tests were carried out before, during and after IMT treatment. Biopsies and smears for staining with the Zichl-Neelsen technique were taken before and after treatment had been completed. Blood samples were taken and intradermal tests applied before each successive IMT dose.
Intradermal tests. During follow up, patients were injected intradermally with 0.1 ml of a suspension of standard integral lepromin (160 x 106 bacilli/ml) and with 0.1 ml of a soluble low molecular weight extract (< 30 kDa) from purified M. leprae (SA). Tests with lepromin were read at 30 days; tests with SA at 48 hr.
Bacterial index. The bacterial index (BI) reflects the density of leprosy bacilli in smears taken from lesions or apparently healthy skin. The smears were graded from 0+ to 6+ according to the WHO logarithmic scale after Zichl-Neelsen staining and examination under oil immersion at 100x magnification (9).
Lymphocyte transformation test (LTT). Mononuclear cells were obtained from 20 ml of heparinized blood and processed with antigens to determine proliferation as previously described (14). The M. leprae antigens used were 20 µ l of SA containing 250 µ g of protein/ml and 20 µ l of purified whole bacilli from a suspension containing 60 x 106 bacilli/ml. BCG (Connaught Laboratories, Willowdale, Ontario, Canada) was heat-killed and 20 µ l of a suspension containing 0.18 mg/ml was added to each well. Stimulation indexes were calculated by dividing mean counts per minute (cpm) in the antigen-containing cultures by mean cpm in antigen-free cell cultures.
Serologic assay. IgM antibodies against native PGL-I were measured in an enzyme-linked immunosorbent assay (ELISA) using the method described previously (17). Results of the determinations were expressed as an ELISA index; an index of 10 corresponds to the value of the positive control (pooled sera from untreated LL patients). Values greater than 2 were considered positive.
IMT. IMT was administered to all lepromin-negative patients, most of whom were multibacillary. Two to 10 doses of a mixture of purified, heat-killed M. leprae (6 x 108
bacilli) and 0.02 and 0.2 mg of live BCG were administered in a period of 1 to 3 years, as previously described (4). The BCG dose was based on PPD skin-test reactivity; the number of IMT doses depended on the type of leprosy and the response obtained.
RESULTS
Patients. Figure 1 shows the different types of patients [ LL, BL, indeterminate (IL) and BT ] and their distribution according to the number of vaccine doses applied.
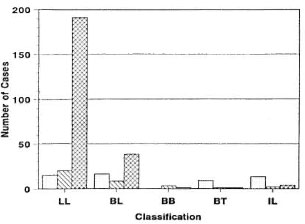
Fig. 1. Patients with different clinical forms of leprosy [LL, BL, BB, indeterminate (IL) and BT] distributed according to the number of vaccine doses administered: < 5 doses  ; 5-7 doses
; 5-7 doses  , 8-10 doses
, 8-10 doses  .
.
Bacterial index. Figure 2A shows the distribution of mean Bis before initiating IMT in relation to the different clinical forms of leprosy. There was a peak at 3+ in multibacillary disease. At the end of the observation period, a marked decrease in the bacillary load in LL patients treated with IMT was apparent (Fig. 2B).
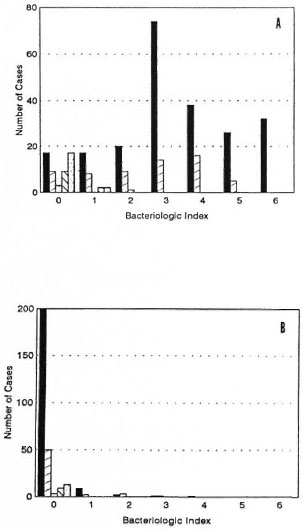
Fig. 2. Distribution of the initial (A) and final (13) average bacterial indexes in relation to the clinical form of leprosy: LL  , BL
, BL  , BB
, BB  , BT
, BT  , indeterminate (IL)
, indeterminate (IL)  .
.
Intradermal tests. As shown in Figure 3, many patients with initial negative skin tests to lepromin became positive during the study. Pre-trial lepromin skin tests were negative in 95% of the multibacillary patients, in spite of the fact that most had received prolonged monotherapy (Fig. 3A). After treatment with MDT and IMT, 49% gave responses of 6 to 10 mm induration with SA and 26% gave reactions > 10 mm (Fig. 3B). As shown in Figure 4A, 98% of the multibacillary patients were initially negative to SA. At the end of the observation period, 39% remained negative, 35% gave reactions between 1 and 10 mm, and 26% gave frankly positive reactions, > 10 mm (Fig. 4B).
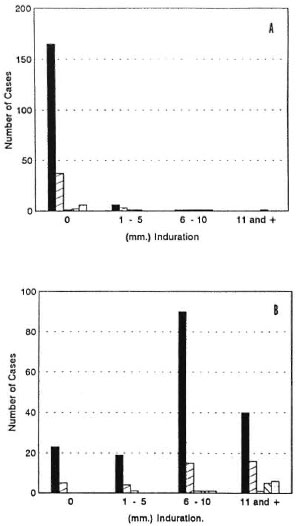
Fig. 3. Distribution of the average induration (mm) of lepromin reactions before (A) and after (B) immunotherapy in patients with: LL  , BL
, BL  , BB
, BB  , BT
, BT  ,and indeterminate (IL)
,and indeterminate (IL)  .
.
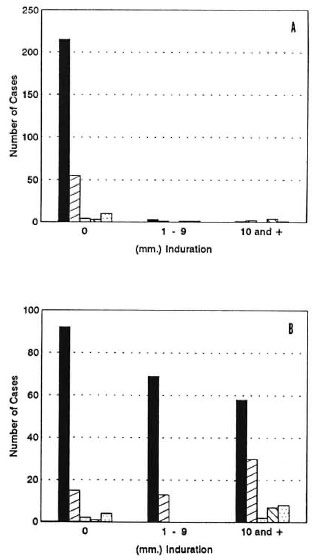
Distribution of the average induration (mm) in skin tests with M. leprae -soluble antigen before (A) and after (B) immunotherapy in different forms of leprosy: LL  , BL
, BL  , BB
, BB  , BT
, BT  , indeterminate (IL)
, indeterminate (IL)  .
.
Serologic assay. The results shown in Figure 5 indicate a very marked and sustained decrease in the level of IgM antibodies against PGL-I in most patients during the course of the study. Pre-trial antibody indexes were 0-2 in 62 of 155 patients, 2.1- 5 in 53, 5.1-8 in 31, and > 8 in 9 patients (Fig. 5A). After treatment with IMT, 133 of 155 patients had ELISA indexes lower than 2 (Fig. 5B). The mean initial ELISA index in 64 LL patients was 5.2 and the final average index in this group was 2.07; in BL patients the mean initial index of 5.11 dropped to 1.11 (Table 1). This marked and sustained decrease was statistically highly significant (p < 0.0001, Student's t test for paired samples) in both groups.
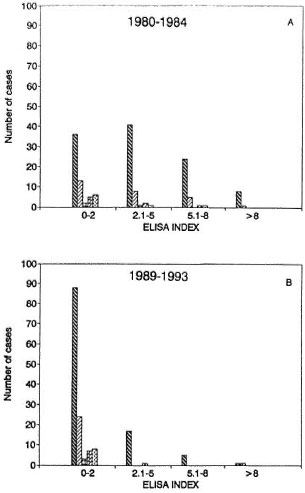
Fig. 5 Levels of antibodies against PGL-I before (A) and after (B) immunotherapy in patients with different forms of leprosy: LL  , BL
, BL  ,BB
,BB  ,BT
,BT  indeterminate (IL)
indeterminate (IL)  .
.
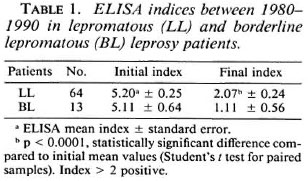
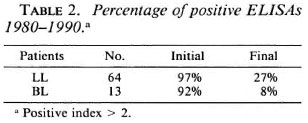
As shown in Table 2, 97% of the LL patients had initial ELISA indexes greater than 2; 27% were still positive at the end of the study. In the BL group, 92% had initial indexes greater than 2; 8% remained positive at the end of the observation period.
LLTs. An increase in the proliferative response of peripheral blood mononuclear cells toward mycobacterial antigens was observed in many patients who received IMT together with MDT. In most, the first reactions to become positive were against BCG and, subsequently, they became reactive to antigens of M. leprae. As shown in Table 3, 61 %, 5% and 0% of LL patients gave an initial positive LTT (stimulation index > 2) against BCG, SA and whole M. leprae, respectively; positivity increased to 87%, 34% and 16%. In the BL group, initial positive values of 46%, 8% and 0% to the same antigens increased to 85%, 38% and 31%.
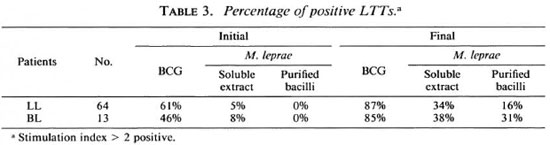
Mean control cpm in antigen-free cultures ranged from 100-300; positive stimulation indexes to BCG showed a broad range from 2 to 50, while positive indexes to M. leprae antigens showed a narrower range of 2 to 10.
DISCUSSION
Most patients who submitted to IMT with the combined M. leprae-BCG vaccine in this study had lepromatous leprosy. They required more IMT doses (8 to 10) to induce favorable immunological changes than did the paucibacillary patients, who received fewer than 5 doses.
As would be expected, the initial average bacillary load was relatively high in the LL group, in spite of the fact that many had received monotherapy for as long as 20 years. Most patients became persistently negative during the course of treatment with MDT and IMT.
Intradermal tests measuring pre-existing delayed-type hypersensitivity and the induction of a granulomatous response to M. leprae also were negative initially in the vast majority of the LL and BL patients, in spite of previous chemotherapy. These values serve as their own internal controls to evaluate sulfone chemotherapy-related lepromin conversion. MDT had been completed by 1986 in 60% of these patients. After IMT plus MDT, 75% of these patients had positive lepromin reactions of which 26% were 11 mm in diameter or greater, demonstrating stable, persistent conversion. In contrast, Waters, el al. (18) have reported spontaneous conversion to lepromin positivity (reactions of 3 to 4 mm) in 54% of a group of lepromatous patients after an average of more than 20 years of chemotherapy; patients who had received MDT more recently were included in their study.
The absence of significant cell-mediated immunity in patients submitted to chemotherapy alone was also suggested by the absence of reversal reactions in 238 patients who received MDT from 1979 to 1985 in our Institute, although 80% to 90% appeared to be clinically cured; 54% of the LL patients and 83% of the BL patients receiving IMT gave reversal reactions which were mild and required no treatment with very rare exceptions (5). During IMT, a majority of 60 patients showed histopathological changes compatible with more resistant forms of leprosy (11).
PGL-I, characterized by Brennan and coworkers in 1980-1982 (7), is a highly specific antigen of M. leprae. Serological assays, which are usually used to detect IgM antibodies against the native antigen or synthetic neoantigens with the same determinants, have been very widely used in recent years (2,4). Most untreated multibacillary patients have high levels of IgM antibodies against PGL-I. Undoubtedly the low initial levels observed in some patients in this study at least partially reflect the fact that they had previously received many years of sulfone monotherapy.
In this study, antibodies against PGL-I decreased markedly in 87% of the patients and have remained negative up to 5 years after MDT-IMT. Similar results showing a rapid and consistent decline in sequential measurements of IgM antibodies against PGL-I in serum samples taken during the first 5 years of therapy have been reported previously (12). Undoubtedly, antibody levels are inversely related to bacterial load in most patients.
Lymphocytes from more than 60% of the lepromatous patients in this study proliferated in the presence of BCG in in vitro assays before the initiation of IMT, but none responded to whole M. leprae and only 5% responded to soluble antigen extracted from M. leprae. Activity to BCG increased after IMT and, much more significantly, more than a third became reactive to M. leprae antigens.
Analysis of the skin test and LTT data clearly suggests that the lepromin reaction constitutes a more sensitive measure of immunological conversion than LTT with whole bacilli, undoubtedly because of the prolonged persistence of the bacillary antigen and accompanying amplification of in vivo reactivity by recruitment of other cells, sustained lymphokine production, granuloma formation and other factors. LTT showed a somewhat higher percentage of positive tests to M. leprae soluble extract than skin tests with a low molecular weight fraction of the soluble extract, perhaps because of the greater number of active components in the total extract used in LTT tests.
Seventy-one percent of the IMT-treated patients in this study gave final ELISA indexes < 2 and LTT stimulation indexes > 2, suggesting an appropriate immunological response associated with resistance to M. leprae. High antibody levels persisted in 6.8% of the LL patients and increased in 3.3% (data not shown). These values appear to coincide with a small group of patients who never respond clinically and others who may have persisting bacilli in visceral lesions or other sites undetected in examinations of the skin.
The results presented in this study show persistent changes in cell-mediated immunity after the treatment of multibacillary patients with MDT and IMT. We have not observed similar changes in patients receiving sulfone monotherapy alone (initial values presented in this study) nor in patients who have received MDT alone, but the latter group has not been followed for a similar period of time. We are unaware of any reports in the literature indicating significant changes in cell-mediated immunity subsequent to MDT in significant numbers of multibacillary leprosy patients during a 10year period.
Acknowledgment. This research was supported by grant No. SP01 A1-26491-03 from the National Institutes of Health, U.S.A. , and by grants from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and el Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICIT) of Venezuela. The authors thank all technical personnel and public health inspectors who collaborated in this study. We also thank Wilman Clark for his help in data management and preparation of the figures.
REFERENCES
1. BACH, M.-A., WALLACH, D., FLAGEUL, B., HOFFENBACH, A. and COTTEN-T, F. Antibodies to phenolic glycolipid-I and to whole Mycobacterium leprae in leprosy patients; evolution during therapy. Int. J. Lepr. 54(1986)256-267.
2. BRENNAN, P. J. and BARROW, W. W. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int. J. Lepr. 48(1980)382-387.
3. CHATURVEDI, R. M., CHIRMULE, N. B., YELLAPURKAR, M. V., SHAIKH, S. V. and MADHAR, G. D. Effects of ICRC antileprosy vaccine in healthy subjects. Int. J. Lepr. 55(1987)657-666.
4. CONVIT, J., ARANZAZU, N., ULRICH, M., PINARDI, M . E., REYES, O. and ALVARADO, J. Immunotherapy with a mixture of Mycobacterium leprae and BCG in different forms of leprosy and Mitsuda-negative contacts. Int. J. Lepr. 50(1982)415-424.
5. CONVIT, J., ULRICH, M., ARANZAZU, N., CASTELLANOS, P. L., PINARDI, M. E. and REYES, O. The development of a vaccination model using two microorganisms and its application in leprosy and leishmaniasis. Lepr. Rev. 57(1986)263-273.
6. HAREGEWOIN, A., GODAL, T., MUSTAFA, A. S., BE-LEHU, A. and YEMANEBERHAM, T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature 303(1983)342-344.
7. HUNTER, S. W . and BRENNAN, P. J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J. Bacteriol. 147(1981)728-735.
8. LEE, S. P., STOKER, N. G., GRANT, K. A., HANDZEL, Z. T., HUSSAIN, R. M., MCADAM, K. P. W. J. and DOCKRELL , H. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect. Immun. 57(1989) 2475-2480.
9. LEIKER, D. L. and MCDOUGALL, A. C. Technical Guide for Smear Examination for Leprosy. Amsterdam: Leprosy Documentation Service, 1983, pp. 7-29.
10. MEHRA, V., BRENNAN, P. J., RADA, E. C, CONVIT, J. and BLOOM , B. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature 308(1984)194-195.
11. MEYERS, W. M., MCDOUGALL, A. C, FLEURY, R. N., NEVES, R., REYES, O. and BINFORD, C. H. Histologic responses in sixty multibacillary leprosy patients inoculated with autoclaved Mycobacterium leprae and live BCG. Int. J. Lepr. 56(1988)302-309.
12. MILLER, R. A., GORDER, D. and HARNISCH, J. P. Antibodies to phenolic glycolipid-I during long term therapy; serial measurements in individual patients. Int. J. Lepr. 55(1987)633-645.
13. MODLIN, R., GERSUK, G. M., NELSON, E. E., PATENGALE, P. K., GUNTER, J. R., CHEN, L., COOPER, C. L., BLOOM, B. and REA, T. H. T-lymphocyte clones from leprosy skin lesions. Lepr. Rev. 57(1986)143-147.
14. RADA, E., CONVIT, J., ULRICH, M., GALLINOTO, M. E. and ARANZAZU , N. Immunosuppression and cellular immunity reactions in leprosy patients treated with a mixture of Mycobacterium leprae and BCG. Int. J. Lepr. 55(1987)646-650.
15. RADA, E., SANTAELLA, C, ARANZAZU, N. and CONVIT , J. Preliminary study of cellular immunity to M. leprae protein in contacts and leprosy patients. Int. J. Lepr. 60(1992)189-194.
16. RIDLEY, D . S. and JOPLING, W . H. Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-276.
17. ULRICH, M., SMITH, P., SAMPSON, C, ZUNIGA, M., CENTENO, M., GARCIA, V., MANRIQUE, X., SALGADO, A. and CONVIT, J. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela; epidemiological observations and a prospective study of the risk of leprosy. Int. J. Lepr. 59(1991)405-415.
18. WATERS, M. F. R., RIDLEY, D. S. and LUCAS S. B. Positive Mitsuda lepromin reactions in long-term treated lepromatous leprosy. Lepr. Rev. 61(1990)347-352.
1. M.S.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
2. Ph.D.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
3. M.D.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
4. M.S.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
5. B.S.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
6. Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
7. M.S.; Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
8. M.D., Instituto de Biomedicina, Apartado 4043, Caracas 1010A, Venezuela.
Received for publication on 31 March 1994;
Accepted for publication in revised form on 28 July 1994.