- Volume 62 , Number 4
- Page: 559–67
Epidemiologic characteristics of leprosy reactions
ABSTRACT
An 8-year prospective study of a cohort of 176 newly diagnosed leprosy patients was conducted to examine the possible influence of age, sex, multidrug therapy (MDT), and duration of illness on the risk of cither type 1 or type 2 reactions. Patients were enrolled over a 5-ycar period (1984-1989) and followed for a minimum of 3 years. All reactions studied were severe enough to warrant hospital admission. Overall, 45% of this cohort developed a reaction; 32% of patients considered at risk developed type 1 reactions, and 37% of patients considered at risk developed type 2 reactions. Despite the predominance of men among the leprosy patients, type 1 reactions occurred with significantly greater frequency in women, and did not appear to be influenced by age of onset of leprosy. Individuals experiencing one type 1 reaction were not likely to experience a recurrence, suggesting that the immunologic mechanisms of this reaction may be limited or regulated by genetic or immunologic factors.Type 2 reactions, on the other hand, occurred with equal frequency in both males and females, but were highly associated with onset of leprosy in the second decade of life. Individuals who experienced type 2 reactions often had one or more recurrence of the reaction. No increased risk was seen for cither reaction with longer duration of leprosy or longer duration of treatment. The mechanisms by which these differences relate to the pathogenesis of leprosy reactions remains unclear, but future studies of clinical and immunological parameters of leprosy reactions may benefit f rom stratification of data by gender and age of onset of leprosy in addition to the routine grouping of results by leprosy classification.
RÉSUMÉ
Une étude prospective d'une durée de 8 ans dune cohorte de 176 malades de la lèpre nouvellement diagnostiqués a été réalisée pour étudier l'influence possible de l'âge, du sexe, de la polychimiothérapie (PCT) et de la durée de la maladie sur le risque de développer des réactions de type 1 ou de type 2. Les patients ont été enrôlés dans l'étude au cours d'une période de 5 ans ( 1984-1989) et suivis pour un minimum de 3 ans. Toutes les réactions étudiées étaient suffisamment sévères pour mériter l'hospitalisation. Au total, 45% des patients de cette cohorte ont développé une réaction; 32% des patients considérés à risque ont développé une réaction de type 1 et 37% des patients considérés à risque une réaction de type 2. En dépit de la prédominance masculine parmi les malades de la lèpre, les réactions de type 1 sont apparues avec une fréquence significativement plus élevée chez les femmes, et ne paraissaient pas être influencées par l'âge à l'acquisition de la lèpre. Les personnes chez qui survenait une réaction de type 1 avaient peu de risque de présenter une récidive, ce qui suggère que les mécanismes immunologiques de cette réaction peuvent être limités ou contrôlés par des facteurs génétiques ou immunologiques.Les réetions de type 2 apparaissaient, quant à elles avec une fréquence semblable chez les hommes et les femmes, mais il y avait une forte relation avec le développement de la lèpre au cours de la deuxième décennie de vie. Les personnes qui avaient eu une réaction de type 2 avaient souvent une ou plusieurs récidives de la réaction. On n'a pas observé d'augmentation du risque d'aucun des types de réaction pour une lèpre de longue durée ou un traitement de longue durée. Les mécanismes par lesquels ces différences sont reliées à la pathogénèse des réactions lépreuses reste peu clair, mais des études futures de paramètres cliniques et immunologiques des réactions lépreuses pourraient bénéficier de la stratification des données par sexe et âge à l'acquisition de la lèpre, en plus du groupement habituel des résultats selon le type de lèpre.
RESUMEN
Se hizo un estudio prospectivo en un grupo de 176 pacientes con lepra recién diagnosticada apra examinar la influencia de la edad, el sexo, el tratamiento con poüquimioterapia (PQT) y la duración de la enfermedad, en el desarrollo de reacciones leprosas de los tipos 1 ó 2. Los pacientes se enrolaron en el estudio a lo largo de 5 años (1984- 1989) y se estudiaron durante un periodo mínimo de 3 años. Todas las reacciones leprosas estudiadas fueron lo suficientemente severas como para justificar la hospitalización de los pacientes. El 45% de los pacientes desarrollaron algún tipo de reacción; 32% de los pacientes considerados en riesgo desarrollaron reacciones del tipo 1, y 37%, reacciones del tipo 2. No obstante el predominio de hombres en el grupo de pacientes, las reacciones de tipo 1 fueron más frecuentes entre las mujeres. Las reacciones no estuvieron asociadas con la edad de aparición de la enfermedad. Los individuos con reacción de tipo 1 no fueron propensos a experimentar recaídas, sugiriendo que los mecanismos inmunológicos de esta reacción pueden estar sujetos a regulación por factores genéticos o inmunológicos.Por otro lado, las reacciones de tipo 2 ocurrieron con igual frecuencia tanto en hombres como en mujeres, pero fueron más frecuentes en los casos donde la enfermedad apareció en la segunda década de la vida. Los individuos que desarrollaron reacciones de tipo 2, frecuentemente presentaron una o más recurrencias reaccionales. El riesgo de aparición de la reacción leprosa no estuvo asociado ni con una mayor duración de la enfermedad, ni con un mayor tiempo de tratamiento de la misma.
Los mecanismos de relación entre las reacciones leprosas y los parámetros analizados permanecen obscuros, pero los estudios clínicos e inmunológicos de las reacciones leprosas, podrían, en un futuro, beneficiarse si los datos se presentaran en forma estratificada en función del sexo del paciente y de la edad de aparición de la enfermedad.
Leprosy reactions are acute emergencies in the otherwise generally indolent course of human infection with Mycobacterium leprae, and account for substantial morbidity, hospitalization, and difficulty in clinical management of leprosy (2,22,24). Two types of reaction are clinically differentiated: type 1 reactions, often called "reversal reactions" (RR), appear as exacerbations of preexisting lesions, and arc accompanied by systemic symptoms; type 2 reactions, otherwise known as erythema nodosum leprosum (ENL), arc characterized by the sudden appearance of crops of tender, erythematous nodules in areas of the body that had not necessarily been involved previously (11,16).
Although leprosy reactions have been the subject of several clinical and laboratory investigations (2,9,12,14,22), their etiologies arc unknown and their pathogenesis poorly understood. Several studies indicate that type 1 reactions are associated with activation of the cellular immune system (1,27,30,34) but the stimulus for this activation is not known. Type 2 reactions are generally considered to be immune complex phenomena (32), although much evidence suggests that circulating complexes are not responsible (15,29) and available data are consistent with but do not convincingly implicate tissue-derived complexes (4,20).
To learn more about the epidemiology of leprosy reactions, we therefore examined their occurrence in a longitudinal, prospective study of newly diagnosed leprosy patients. We sought particularly to determine whether temporal variables in the early course of the disease (including age of onset, duration of illness prior to reaction, age of first reaction, etc.) were associated with a risk of cither reaction.
MATERIALS AND METHODS
Study subjects were enrolled from those patients presenting for initial diagnosis at the clinics of the McKean Rehabilitation Institute, Chiang Mai, Thailand. Patients who had been previously diagnosed and treated at other facilities were excluded; all newly diagnosed patients were considered eligible for study except those occasional patients from "hill tribes," for whom no suitable translators were available or who lived at great distances and could not be suitably followed. Patients with reactions at the time of diagnosis were included in the study. Otherwise, the outcome endpoint was considered to be the first type 1 or type 2 reaction experienced by each patient.
Enrollment began in the fall of 1984 and continued until the fall of 1989. Follow-up continued through fall 1992, so that each patient was followed a minimum of 3 years. Initial histories by both the research team and the attending physicians documented the patient's year of birth and age at onset of first leprosy symptoms. A representative lesion was biopsied in each patient; each was classified as to clinical category of leprosy, according to the five-part scale of Ridley and Jopling (19), based on clinical and histologic criteria.
All patients were given standard multiple drug therapy (MDT): 100 mg dapsone daily, 100 mg clofazimine every other day, and 1.2 g of rifampin monthly in two consecutive doses (if multibacillary), or dapsone and rifampin (if paucibacillary). Treatment was usually continued until patients were skin-smear negative for multibacillary (MB) patients, and for at least 6 months for paucibacillary (PB) patients. Patients were seen at regular intervals, usually every 1 or 2 months, and were encouraged to come to the clinic or hospital if they experienced worsening of local or systemic symptoms.
All reactions documented in this study were severe enough to warrant hospital admission. An attempt was made to biopsy all reaction lesions. However, due to the different durations of reactions before diagnostic presentation and the lack of uniform criteria for histologic diagnosis, diagnosis of reactions was ultimately based on the clinical appearance of characteristic lesions of type 1 or type 2 reactions. Type 1 reactions were diagnosed when existing borderline lesions became inflamed, i.e., erythematous, swollen or raised, and tender, combined with pain and/or tenderness of involved peripheral nerves. The degree of systematic involvement varied, but most had low-grade fever with or without malaise. In patients who originally presented without type 1 reactions, these findings represented definite changes from the patient's previous status. Type 2 reactions were diagnosed when small, inflammatory, red, swollen and highly tender nodules developed suddenly in the skin or subcutis, accompanied by fever and malaise. Most of these patients also had a mild leukocytosis and acute neuritis, with one or more of the following: arthritis, orchitis, iritis, myalgia, and peripheral edema.
Patients with reactions were given standard treatment with corticosteroids and/or thalidomide as considered appropriate in individual cases by the attending physicians, and their MDT regimen for leprosy was not interrupted. All medication, as well as the progression and regression of signs and symptoms, were charted on flow sheets especially designed for the study. Immunologic studies of reactions in the patients reported here have been published previously (3,17,26,27).
Statistical comparisons of proportions were made using the two-tailed chi-squared test with Yates' correction for continuity.
RESULTS
A total of 176 patients were prospectively enrolled in the study between 1984 and 1989. The distribution of patients by classification of leprosy was as follows: 55 LL, 64 BL, 16 BB, 31 BT, 9 TT, and 1 indeterminate. When this distribution was further categorized by sex (Fig. 1), the preponderance of males and of MB patients is evident, as previously documented in this population. One patient in our cohort, a 28-ycarold male with LL disease, died soon after diagnosis of an apparent dapsone reaction, and he is not included in the calculations of the incidence of leprosy reactions since the duration of follow up was only a few months. None of the remaining 175 patients was lost to follow up during the study.
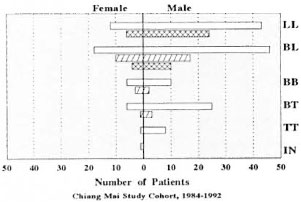
Fig. 1. Distribution of reactions among leprosy patients. The distribution of incident, medically observed reactions is indicated in the cohort of 176 new patients according to sex and leprosy classification (IN = indeterminate).  = All patients of each classification in the cohort;
= All patients of each classification in the cohort;  type 1 reaction;
type 1 reaction;  = type 2 reaction.
= type 2 reaction.
The study cohort equalled 45% (176) of the 388 new, untreated patients registered at McKean during the study interval, including 79% of 171 MB (LL-BB) patients and 32% of 97 BT patients. Twenty-three of the new patients at McKean were indeterminate, of whom one was enrolled in our study. This sample thus may underestimate reactions in BT patients, but approximates the total population of new MB patients in this population, whose greater acceptance of a brief hospital admission (for related immunologic studies) may have led to increased enrollment. Despite the different proportions of study volunteers in each group, a review of medical records of participating and non-participating patients in each clinical group revealed no differences in age, gender, occupation, area of residence, or severity of leprosy, except for patients excluded because of inaccessibility to follow up.
Of the 175 patients followed, 79 (45%) developed one or more reaction; 35 developed type 1 reactions and 44 developed type 2 reactions. Two patients developed both type 1 and type 2 reactions. Two additional patients who developed severe neuritis not associated with typical reaction skin lesions were excluded from analysis because the basis for their neuritis could not be clearly determined.
The incidence of reactions in this cohort of new patients, unadjusted for duration of follow up, indicates the association of type 1 reactions with borderline patients, and of type 2 reactions with LL and I3L patients (Table 1). Thirty-two percent of patients at risk for type 1 reactions (i.e., borderline patients, BL-BB-BT) developed this reaction, and 37% of patients at risk for type 2 reactions (i.e., MB patients, LL and BL) developed this reaction.
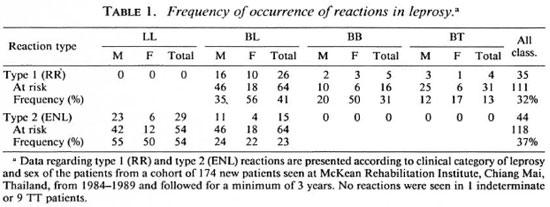
Also noteworthy is the greater risk for type 1 reactions in female patients, 47% vs 26% overall, which appears independent of clinical category (Table 1). Analysis of the frequency of type 1 reactions by age at onset of leprosy shows that the increased occurrence is seen in women with onset of leprosy at all ages (Table 2). These findings were not confounded by duration of leprosy prior to diagnosis (data not shown).
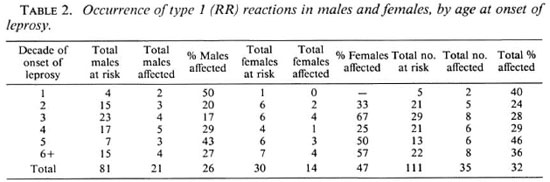
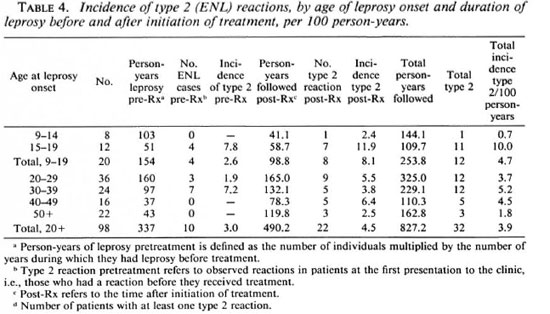
The development of type 2 reactions appears to be predicted by the age of onset of leprosy rather than by patient age at the time of the reaction (Table 3). Unadjusted for duration of follow up, patients whose first symptom occurred during adolescence had a much greater frequency (71%) of experiencing a type 2 reaction than patients whose onset of leprosy occurred after adolescence (p <0.05, chi-squared with Yates' correction). That the association of age of leprosy onset and the occurrence of type 2 reactions was not an artifact of differential follow up is demonstrated by person-year incidence calculations: the risk of type 2 reactions for persons who had leprosy onset before age 20 was greater than for persons who had leprosy onset in adulthood, a difference that appeared to be largely due to greatly increased risk in persons with onset between 15 and 19 years of age (10.0 cases per 100 person-years of follow up, Table 4).
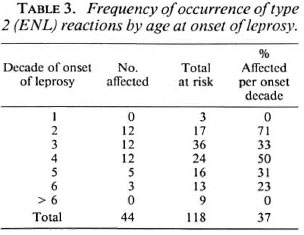
The increased frequency of type 2 reactions in patients with leprosy onset in the second decade of life was not due to longer duration of illness prior to diagnosis (Fig. 2) nor to total duration of illness (prediagnosis plus postdiagnosis follow up, Table 4). In fact, a shorter disease course before diagnosis in persons with leprosy onset before age 20 was associated with development of a type 2 reaction (Fig. 2).
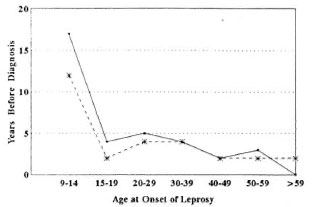
Fig. 2. Mean duration of leprosy before diagnosis and treatment (MDT). The duration of leprosy (in years) was determined by careful history at the time of diagnosis; MDT was initiated at diagnosis. Dates are shown according to age at onset of leprosy. - = patients who developed ENL; --- = patients who did not develop ENL. The two curves are not statistically different.
The MDT regimen used appears to have had little effect on the occurrence of reactions (Table 4) when comparing patients who had reactions on admission (i.e., before treatment) to patients experiencing reactions only after starting treatment. No difference was seen in the occurrence of type 1 reactions in these two groups of patients. However, type 2 reactions were prevalent in 14/118 (12%) of at-risk patients on admission, but after starting MDT type 2 reactions developed in 30 (25%) of these atrisk patients (Table 4). These data provide no evidence that treatment either protects or precipitates type 2 reactions in patients at risk.
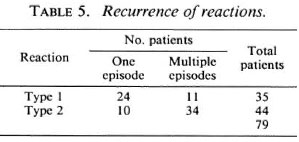
Recurrence of type 1 and type 2 reactions followed different patterns. The majority of patients (34/44) with type 2 reactions had multiple episodes of reaction, while only a minority of patients (11/25) with type 1 reactions had multiple episodes (Table 5). This difference was statistically significant (p <0.001). In addition, among patients experiencing multiple episodes of reaction, type 1 reactions tended to recur only one or two times, but type 2 reactions often recurred more than four times (data not shown). There was no difference in the pattern of recurrence when comparing patients who developed reactions prior to or subsequent to treatment (data not shown).
DISCUSSION
A potential concern in any prevalence or prospective study based on the sampling of persons in different exposure categories is that sampled persons may not be representative of all persons sampled and unsampled. In such circumstances, risk factors associated with selection for participation could be identified erroneously as being linked to disease outcome. Although this type of sampling bias cannot be entirely excluded in our prospective study, it is reassuring that except for residence differences in the persons excluded from participation because they lived in inaccessible areas, the demographic characteristics of participating and non-participating patients in both PB and MB disease categories did not differ. Moreover, despite the expected differences in participation rates between these clinical groups, both the total sample (176 persons) and the sub-samples (64 and 118 persons, respectively) were of ample size.
The overall incidence and risk of reactions in this study is comparable to those reported in previous studies of large numbers of patients receiving MDT in Ethiopia (2), Zaire (9), and India (6,12), although overall figures vary widely due to differences in methodology and racial and geographic origins. Our data, however, characterize type 1 reactions as occurring disproportionately in females independent of age of leprosy onset, and type 2 reactions as occurring equally in males and females and being associated with pre-adult onset for both.
That women have a greater risk of type 1 reaction than men in this population is particularly interesting since the overall incidence of leprosy is much greater for men than women. The well-documented increased incidence of leprosy in men has never been explained; endocrine influences have been proposed (7), but no studies have thus far confirmed or rejected this hypothesis.
Although women are disproportionately represented among patients with type 1 reactions in this study, the basis for this finding is unclear. That this association appears to cross all age groups, from adolescence to old age, may be interpreted as an argument against an endocrine influence on immunologically mediated events in leprosy. In addition, we found no association of age of onset of leprosy with occurrence of type 1 reactions, although the characteristically long incubation period of leprosy might allow for estrogen exposure of older women who had had initial infection before menopause. We did not record data on postmenopausal hormonal treatment in our female subjects, but believe such treatment to be uncommon in this patient population.
Various lines of evidence have clearly associated type 1 reactions with spontaneous activation of the cellular immune system, as indicated by elevated levels of circulating Tac peptide and increases in CD4+ lymphocytes during the reactions (13,30). Some studies have suggested that a major source of circulating Tac peptide may be the lymphocytes in the skin lesions themselves (27), but the stimulus for activation and the immunoregulatory mechanisms involved in the recruitment or proliferation of CD4 + cell subsets have not been elucidated. The results of this study indicate that the immunologic evidence may be more informative if interpreted in the light of a possible endocrine influence on either systemic or dermal immune mechanisms (21). Another possibility is that the mechanisms involved in type 1 reactions may share some common determinants with other autoimmune phenomena to which women are predisposed (31).
The observation that type 1 reactions typically occur only once (or a very few times) suggests that in each individual only a limited degree of "adjustment" in the immune response to M. leprae can take place. If so, the permissible range of such change might be determined or limited by genetic or immunologic factors which can be examined in future studies.
The most interesting observation concerning type 2 reactions in this study is the apparently increased frequency of these reactions in individuals whose onset of leprosy symptoms occur before adulthood. Other studies have noted that type 2 reactions arc more often seen in patients between 20 and 40 years old (6,25), but the association with onset of leprosy in adolescence previously has not been noted. Any hypothesis to explain this observation must account for the fact that type 2 reactions occur after a variable duration of 4 months to 3 or more years after the onset of leprosy. If the later occurrence of type 2 reactions is related to onset during adolescence, the initiating events must take a long and variable time to become manifest.
Davey and Schenk (7) long ago cited studies noting that puberty is associated with increased risk of relapse of leprosy and of the development of lepromin sensitivity. The association of puberty with increased susceptibility to and mortality from tuberculosis is also well documented (18), as is the increase in the incidence of systemic lupus erythematosus in the decade following puberty (31). These clinical and epidemiologic phenomena remain unexplained. Neuroendocrine-immune interactions have recently attracted interest (8,21), as have their possible effects on immunity to mycobacteria (33), and the effects of infection-induced cytokines on neuroendocrine function (23,28,33). The possible role(s) of the changes at puberty have not received as much attention, however, as the more obvious mechanisms related to adrenal function.
In addition to the major endocrine changes that occur in both sexes during puberty, this also may be an important phase in the maturation of the immune system. This has not been the subject of substantial research, however, and little is known about it beyond the oft-repcated maxim that the function of the immune system "peaks" around the time of puberty (l0). Our data do not provide a basis to select from an endocrine or an immune-maturation hypothesis in explaining the pathogenesis of ENL, but suggest that further studies of ENL should consider both possibilities.
The characteristics of both type 1 and type 2 reactions identified in this study may assist in distinguishing between them: type 1 reactions occurred predominantly in women who had onset at all ages, and type 2 reactions were more common in males and females who had leprosy onset in adolescence. Future studies of immunologic parameters of these reactions may benefit from the stratification of data by sex and age of onset of leprosy in addition to the routine classification of results by leprosy classification.
Acknowledgment
We are indebted to the nursing and technical staff of McKean Réhabilitation Instituto for their generous coopération and excellent patient care, and to the staff of the Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand, for providing years of technical and administrative support. Thèse studies were supported by grants from USAID and by a Research Strengthening Grant from the World Health Organization.
REFERENCES
1. BARNETSON, R. ST.C , BJUNE, G., PEARSON, J.M.H. and KRONVALL, G. Cell-mediated and humoral immunity in "reversal reactions". Int. J. Lepr. 44(1976)267-274.
2. BECX-BLEUMINK, M . and BERHE, D . Occurrence of reactions, their diagnosis and management in leprosy patients treated with multidrug therapy; experience in the leprosy control program of the All Africa Leprosy and Rehabilitation Training Center (ALERT) in Ethiopia. Int. J. Lepr. 60(1992)173-184.
3. BHOOPAT, L., SCOLLARD, D.M., THEETRANONT, C, CHIEWCHANVIT, S., NELSON, D.L . and UTAIPAT, U. Studies of human leprosy lesions in situ using suction-induced blisters: cell changes with IgM antibody to PGL-1 and interlcukin-2 receptor in clinical subgroups of erythema nodosum leprosum. Asian Pacific J. Allergy Immunol. 9(1991)107-119.
4. BJORVATN, B., BARNETSON, R.ST.C , KRONVALL, 18.G., ZUHLER, R.H. and LAMBERT, P.H. Immune complexes and complement hypercatabolism in patients with leprosy. Clin. Exp. Immunol. 26(1976)388-396.
5. BROWN, D.H., SHERIDAN, J., PEARL, D. and ZWIL-LIG, B.S. Regulation of mycobacterial growth by the hypothalamus-pituitary-adrenal axis: differential responses of M. bovis BCG-resistant andsusceptible mice. Infect. Immun. 61(1993)4793- 4800.
6. CHOPRA, N.K., AGRAWAL, J.S. and PANDYA, P.G. Reactions in leprosy; a study of 250 patients in a multidrug therapy project, Baroda District, Gujarat, India. Int. J. Dermatol. 29(1990)490-493.
7. DAVEY, T.F. and SCHENCK, R.R. The endocrines in leprosy." In: Leprosy in Theory and Practice. Cochrane, R.G. and Davey, T.F. Bristol: John 23.Wright & Sons, 1964, pp. 190-204.
8. EPSTEIN, F.H. Neuroendocrine-immune interactions. N. Engl. J. Med. 329(1993)1246-1253.
9. GROENEN, G., JANSSENS, L., KAYEMBE, T., NOLLET, 24.E., COUSSENS, L. and PATTYN, S. Prospective study on the relationship between intensive bactericidal therapy and leprosy reactions. Int. J. Lepr. 54(1986)236-244.
10. HIROKAWA, K., UTSUYAMA, M., KASAI, M. and KURASHIMA, C. Aging and immunity. Acta Pathol. Jpn. 42(1993)537-548.
11. JOPLING, W.H. Leprosy reactions. In: Handbook of Leprosy. London: Heinemann Medical Books, 1971, p. 42.
12. LOCKWOOD, D.N J., VINAYAKUMAR, S., STANLEY, J.N.A., MCADAM, K.P.W.J. and COLSTON, M.J. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. Int. J. Lepr. 61(1993)8-15.
13. MODLIN, R.L., GEBHARD, F., TAYLOR, C.R. and REA, T. H. In situ characterization of T lympho cyte subsets in the reactional states of leprosy. Clin. Exp. Immunol. 53(1983)17-24.
14. NAAFS, B. and WHEATE, H.W. The time interval between the start of anti-leprosy treatment and the development of reactions in borderline patients Lepr. Rev. 49(1978)153-157.
15. NG, W.L., SCOLLARD, D.M. and HUA, A. Glomerulonephritis in leprosy: case report and review of the literature. Am. J. Clin. Pathol. 76(1981)67-75.
16. PFALTZGRAFF, R.E. and BRYCESON, A.B. Clinical Leprosy. In: Leprosy. Hastings, R. C, cd. New York: Churchill Livingstone, 1985, pp. 134-176.
17. RANGDAENG, S., SCOLLARD, D.M., SURIYANON, V., SMITH, T., THAMPRASERT, K. and THEETRANONT, C. Studies of human leprosy lesions in situ using suction-induced blisters. Cellular components of new, uncomplicated lesions. Int. J. Lepr. 57(1989)492-498.
18. RICH, A.R. The Pathogenesis of Tuberculosis. Springfield, Illinois: Charles C. Thomas, 1951, p. 237 ff.
19. RIDLEY, D.S . and JOPLING, W.H . Classification of leprosy according to immunity; a five-group system. Int. J. Lepr. 34(1966)255-273.
20. RIDLEY, M. and RIDLEY, D.S . The immunopathology of erythema nodosum leprosum: the role of extravascular complexes. Lepr. Rev. 54(1983)95-107.
21. ROOK, G.A.W., HERNANDEZ-PANDO, R. and LI-GHTMAN, S.L. Hormones, peripherally activated prohormones, and regulation of the Th1/Th2 balance. Immunol. Today 15(1984)301-303.
22. ROSE, P. and WATERS, M.F.R. Reversal reactions in leprosy and their management. Lepr. Rev. 62(1991)113-121.
23. SAPOLSKY, R., RIVIER, C, YAMAMOTO, G., PLOTSKY, P. and VALE, W. Interlcukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 238(1987) 522-524.
24. SEHGAL, V.N . Reactions in leprosy; clinical aspects. Int. J. Dermatol. 26(1987)278-285.
25. SEHGAL, V.N., and SHARMA, V. Reactions in leprosy-a prospective study of clinical, bacteriological, immunological, and histopathological parameters in thirty-five Indians. J. Dermatol. 15(1988)412-419.
26. SCOLLARD, D.M. , BHOOPAT, L., KESTENS, L., VANHAM, G., DOUGLAS, J.T. and MOAD, J. Immune complexes and antibody levels in blisters over human leprosy skin lesions with or without erythema nodosum leprosum. Clin. Immunol. Immunopathol. 63(1992 230-236.
27. SCOLLARD, D.M., SURIYANON, V., BHOOPAT, L., WAGNER, D.K., SMITH, T., THAMPRASERT, K., NELSON, D. and THEETRANONT, C. Studies of human leprosy lesions in situ using suction-induced blisters. 2. Cell changes and soluble interIeukin-2 receptor (tac peptide) in reversal reactions. Int. J. Lepr. 58(1990)469-479.
28. TRACY, K.J. and CERAMI, A. Metabolic responses to cachectin/TNF: a brief review. Ann. N.Y. Acad. Sci. 587(1990)325-331.
29. TUNG, K.S.K., KIM, B., BJORVATN, B., KRONVALL, G., MCLAREN, L.C. and WILLIAMS, R.C. Discrepancy between Clq deviation and Raji cell tests in detection of circulating immune complexes in patients with leprosy. J. Infect. Dis. 136(1977) 216-221.
30. TUNG, K.S.K., UMLAND, E., MATZNER, P., NELSON, K., SCHAUF, V., RUBIN, L., WAGNER, D., SCOLLARD, D., VlTHAYASAl, P., VlTHAYASAI, V., WOROBEC, S. and SMITH, T. Soluble serum interIeukin-2 receptor levels in leprosy patients. Clin. Exp. Immunol. 69(1987)10-15.
31. WALLACE, D.J., HAHN, B.H., QUISMORO, F.P., JR., and KLINENBERG, J. R., EDS. Dubois' Lupus Erythematosus. 4th edn. Philadelphia: Lea & Febiger, 1993.
32. WEMAMBU, S.N.C., TURK, J.L., WATERS, M.F.R. and REES, R.J.W . Erythema nodosum leprosum: a clinical manifestation of the Arthus phenomenon. Lancet 2(1969)933-935.
33. WOLOSKI, B.M.R.N.J., SMITH, E.M., MEYER, W.J., III, FULLER, G.M . and BLALOCK, J.E. Corticotropin-relcasing activity of monokines. Science 230(1985)1035-1037.
34. YAMAMURA, M., UYEMURA, K., DEANS, R.J., WEINBERG, K., REA, T.H., BLOOM, B.R. and MODLIN, R.L. Defining protective responses to infectious pathogens: cytokine profiles in leprosy lesions. Science 254(1991)277.
1. M.D., Ph.D., Laboratory Research Branch, GWL Hansen's Disease Center at Louisiana State University, P. O. Box 25072, Baton Rouge, LA 70894, U.S.A.
2. M.B.B.S., McKean Rehabilitation Institute, Chiang Mai, Thailand.
3. M.D.; Department of Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
4. M.D.; Department of Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
5. M.D., Department of Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand.
6. M.D., Schools of Medicine and Public Health, University of Hawaii at Manoa, Honolulu, Hawaii, U.S.A.
Received for publication on 30 December 1993;
Accepted for publication in revised form on 10 August 1994