- Volume 62 , Number 4
- Page: 568–73
Efficacy of minocycline in single dose and at 100 mg twice daily for lepromatous leprosy
ABSTRACT
A clinical trial of minocycline in a total of 10 patients with previously untreated lepromatous leprosy was conducted in order to evaluate the efficacy of a single, initial, 200 mg dose and 100 mg twice daily of minocycline fora total duration of up to 3 months. Patients improved remarkably quickly. Although single-dose therapy did not result in a significant killing of Mycobacterium leprae, viable M. leprae were cleared f rom the dermis regularly by 3 months of twice-daily therapy, a rate similar to that achieved by minocycline 100 mg once daily. Because more side effects were noted herein than previously with 100 mg daily, we recommend that minocycline, when applied, be administered at 100 mg daily to leprosy patients.RÉSUMÉ
Un essai clinique de minocycline a été conduit sur un total de 10 patients présentant une lèpre lépromateuse non traitée antérieurement, afin d'élvalcur l'efficacité d'une dose unique, initiale, de 200 mg de minocycline, puis de 100 mg deux fois par jour pour une durée totale allant jusqu'à trois mois. Les malades ont montré une amélioration remarquablement rapide de leur état. Bien que la dose unique n'ait pas provoqué une destruction significative des Mycobacterium leprae, les M. Leprae viables etaient regulierement elimines du derme par les trois mois due traitement bi-quotidien, et ce à un taux d'élimination semblable à celui obtenu parla minocycline dmininstréc à la dose de 100 mg une fois par jour. Comme les effets secondaires ont été notés plus nombreux ici qu'auparavant avec une dose de 100 mg par jour, nous recommandons que la minocycline, quand clic est administrée aux malades de la lèpre, le soit à une dose de 100 mg par jour.RESUMEN
Se hizo un estudio sobre la efectividad de la minociclina en un grupo de 10 pacientes con lepra lepromatosa sin tratamiento previo para comparar la eficiencia de una sola dosis inicial de 200 mg de minociclinia con aquella de 100 mg de la droga administrada dos veces al día durante 3 meses. Los pacientes mejoraron marcadamente y de manera rápida. Aunque la terapia con una sola dosis no condujo a una significante destrucción de Mycobacterium leprae, la terapia con dos dosis al día condujo, regularmente, a la eliminación de la bacteria de la piel hacia el tercer mes de tratamiento; un resultado similar al encontrado cuando se administra una sola dosis de 100 mg diarios de minociclina. Debido a que con la administración de 2 dosis de 100 mg diarios de minociclina también se notaron más efectos colaterales que con una sola dosis de 100 mg diarios, se recomienda que la droga se administre a los pacientes con lepra a la dosis única de 100 mg al día.In 1987 we (5) discovered that minocycline, among the tetracyclines, had a unique and consistently bactericidal activity against Mycobacterium leprae -infected mice, and at levels several-fold less than those obtained by standard clinical doses. In subsequent studies the powerful bactericidal activity of minocycline against M. leprae was reconfirmed by ourselves (7,8) and others (12). Additionally, it has been noted that minocycline was active against all M. leprae isolates (now over 20) tested (7), was additive in its activity against M. leprae in mice with other established effective antimicrobial agents (5), and retained activity when given intermittently (7), in a few doses (13), and in single doses both in combination chemotherapy (13) and even alone (18).
In the first clinical trial of minocycline 100 mg daily for previously untreated lepromatous leprosy patients, we (6) found it to afford a consistent and very rapid amelioration of skin lesions and bactericidal activity for M. leprae superior to that found previously for dapsone and clofazimine (14). Subsequent clinical trials in lepromatous leprosy of 100 mg daily minocycline both in Africa (11) and in The Philippines (2) confirmed that in lepromatous leprosy minocycline was highly effective both clinically and microbiologically.
Because the World Health Organization (17) has advocated supervised monthly rifampin for the therapy of leprosy, the efficacy of single doses of other antimicrobials which might be similarly administered is of considerable interest, especially if the goal of future chemotherapy is to yet further shorten the duration required to treat patients. Furthermore, since minocycline is often administered to adults for other bacterial infections in a dosage of 100 mg twice daily, and only 100 mg daily has to date been studied in leprosy patients (6,11), this larger dose warranted evaluation in leprosy patients as well. Therefore, the current clinical trial was designed to determine the efficacy of minocycline when administered in a dose of 100 mg twice daily and to determine whether a single 200-mg dose resulted in the clearance of viable M. leprae from the dermis of affected lepromatous patients.
MATERIALS AND METHODS
For these studies 10 previously untreated multibacillary patients were recruited and their informed consent obtained to participate in this clinical trial at the Regional Ambulatory Hansen's Disease Programs in San Francisco (5) and Los Angeles (5). Trial patients were classified according to the clinico-pathologic method of Ridley and Jopling as having borderline lepromatous (4) or lepromatous leprosy (6). Five of the trial patients were male, and five were female. Patients ranged in age from 17 to 75, averaging 50 years of age. Trial patients were born in several endemic countries: Mexico (5), The Philippines (3), Vietnam (1), and Laos (1).
Trial patients had an initial complete history and physical examination performed, as well as a hemogram, blood chemistry profile, and G-6-PD level. M. leprae from all pretreatment skin biopsies were inoculated into both hind foot pads of groups of mice for evaluation of antimicrobial sensitivity to dapsone (0.0001%, 0.001%, and 0.01% in diet) and minocycline (0.01% in diet). For these purposes 5000 M. leprae per foot pad were utilized as the infecting inoculum. Female BALB/c mice (Jackson Laboratories, Bar Harbor, Maine, U.S.A.) were fed continuously drug-containing diet from the time of infection, and growth of > 105 bacilli/foot pad in pools of four foot pads (two mice) obtained 6 or more months after infection was considered indicative of antimicrobial resistance in treated mice.
Eight trial patients (patients 3-10) were administered a 200-mg initial minocycline dose, followed by no therapy for 1 week, and then minocycline 100 mg twice daily for the subsequent 3-month trial period. Two additional patients (patients 1 and 2) were asked to take minocycline 100 mg twice daily for a 1-month period. At the completion of the trial patients were switched to M DT, generally daily dapsone (100 mg) and daily rifampin (600 mg).
The group of eight patients was clinically evaluated and had routine hemograms and blood chemistries as well as skin biopsies performed from the most active lesions on all clinic visits (prior to therapy and at 1 week, 2 weeks, 1 month, 2 months, and 3 months subsequently). The remaining two patients had these same evaluations performed prior to therapy and 3-4 days, 1 week, 2 weeks, 3 weeks (only one patient), and 1 month after the initiation of treatment. On all clinic visits patients were queried concerning clinical progress, side effects (particularly vertigo and diarrhea), and reactional states. At all clinic visits an assessment of the response to therapy of skin lesions, with particular attention to induration and erythema, was performed.
Also on these clinic visits, skin biopsies were performed, and from these 5000 M. leprae were inoculated into both hind feet of female BALB/c mice (Jackson Laboratories). The viability of M. leprae from these biopsies was determined from pools of four hind foot pads (two mice) harvested 8 months and 12 months subsequently, as well as from individual foot pads (generally 10) harvested 1 year subsequently. In both instances viable M. leprae were considered to be present in the initial inoculum if > 105 M. leprae /foot pad were realized.
RESULTS
Trial patients experienced a consistent and rapid clinical response to minocycline ther apy. Seven days after a sole 200-mg dose of minocycline we noted in one of these patients fully 50% flattening of nodules, and in another patient previous nodularity was already entirely flattened. In yet a third patient we noted profound loss of erythema of the skin lesions and the total disappearance of a previously large, indurated, borderline lesion. Of the eight patients treated with an initial 200-mg dose of minocycline, five had improved clinically by this alone at their initial post-treatment clinic visit 1 week subsequently. By 1 month of treatment all eight patients had significant clinical improvement, and by 3 months skin lesions had entirely resolved in 3 of the 5 patients treated for this duration.
After the 200-mg single dose two patients (nos. 6 and 10), both Filipino females, experienced vertigo, requiring discontinuation of therapy. Two other patients experienced mild vertigo during the early course of the trial, which abated and did not require their discontinuation of therapy. Another patient experienced mild light-head-edness and watery diarrhea only during the first week of treatment. A Mexican patient noted a mild, nonprogressive, generalized hyperpigmentation confined to sun-exposed areas 2 months after the initiation of minocycline, which remained of little concern. The patient was continued on the combination of minocycline and rifampin even after the 3-month duration of this trial. Another patient, a Laotian male, developed a gray-blue skin discoloration to the face 1 month after the beginning of the trial, which began to fade 2 weeks after minocycline was discontinued and had essentially resolved 2 months later.
After 8 weeks of therapy one patient (no. 7) developed a profound reversal reaction of the skin, sufficiently mimicking a possible allergic reaction, such that minocycline was discontinued. During the next 4 months and while being treated with dapsone and rifampin, on two separate occasions this patient experienced even more severe reversal reactions. Another patient (no. 8) developed mild erythema nodosum leprosum (ENL) skin lesions which did not require therapy during the course of the trial. In retrospect this patient had similar lesions several months prior to treatment. It is noteworthy that 4 months after minocycline was discontinued and while on dapsone and rifampin, this patient developed severe skin lesions of ENL associated with high fever, hematuria, and anemia (hematocrit 27%), which required hospitalization and the institution of high-dose corticosteroid and thalidomide therapy for control.
No other side effects of therapy were noted. Sequential hemograms and blood chemistries in the 10 treated patients resulted in absolutely no abnormalities.
By four-foot pools at one (5) or both (5) harvest intervals or by individual foot pad harvests (10), viable M. leprae were found present in pretreatment skin biopsies from each of the 10 trial patients (Tables 1 and 2). In nine patients' pretreatment skin biopsies M. leprae were found sensitive to the lowest level of dapsone (0.0001%). In the case of one patient, mice for these purposes died prematurely, making it impossible to assess dapsone sensitivity. Isolates of M. leprae from each of these 10 patients were inhibited by 0.01% minocycline in the mouse diet.
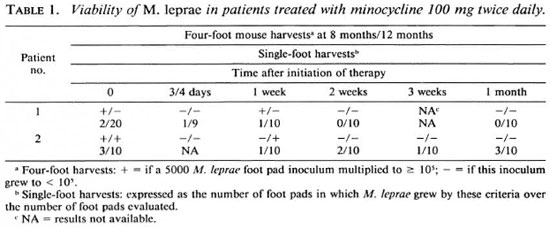
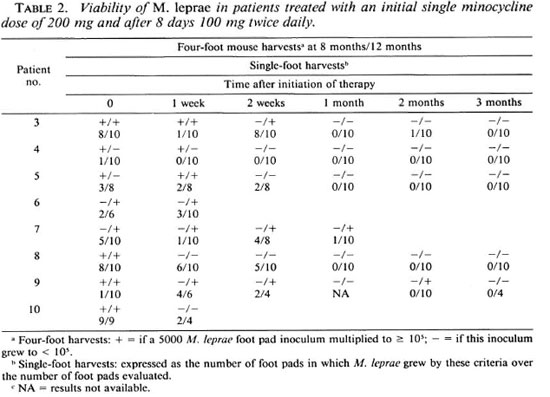
After the single 200-mg dose of minocycline 2 of 8 patients had lost viable M. leprae as determined by the absence of demonstrable bacterial growth in four-foot pad pools taken at both harvest intervals (Table 2). However, even these two patients (nos. 8 and 10) still retained viable M. leprae, although in a smaller proportion than in pretreatment specimens, as determined by the results' of harvests of individual foot pads. In fact, none of the eight patients had lost viable M. leprae by single-dose therapy in all individual foot pads analyzed and in the four-foot pad pools (Table 2). It should be noted, however, that the proportion of individual foot pads which demonstrated viable M. leprae after single-dose therapy was decreased in 6 of these 8 patients (Table 2); only one of these (patient no. 3) was found statistically significant by the twotailed Fisher's exact test.
It was found by the biopsy performed 1 month after the initiation of therapy that viable M. leprae had largely disappeared from the skin, only 2 of 8 patients still harboring these by the methods utilized in this study (Tables 1 and 2). By 2 months a similar finding was noted: only 2 of 5 patients still harbored viable bacilli. Interestingly, both of these did not demonstrate viable bacilli at the 1 -month biopsy interval (Table 2). By 3 months' therapy none of 5 patients had viable M. leprae detected either by fourfoot pools or individual foot pad harvests (Table 2).
DISCUSSION
In this clinical trial of minocycline in lepromatous leprosy, it was once again found to rapidly clear skin lesions and viable acidfast bacilli from the dermis. It was noteworthy, also, that only 1 of these 10 trial patients developed ENL, that single patient having mild skin lesions alone but developing profound ENL with systemic manifestations only after the trial was completed and while receiving dapsone and rifampin. In this study side effects, particularly those of dizziness and skin discoloration, were more problematic than in our earlier study of minocycline at 100 mg daily. The two trial patients who experienced significant dizziness after a single, initial 200-mg dose of minocycline and, hence, were not asked to take the additionally planned minocycline therapy were both women. It has been found previously that side effects of minocycline of equivalent doses, perhaps owing to the significantly higher levels obtained in female patients or their increased percentage of body fat, were more commonly encountered in women than in men (3).
The resolution of leprous erythema and induration found in these patients was, indeed, from our experience considered most impressive. After only a single dose of 200mg minocycline a clinically apparent amelioration of lesions was evident in 5 of 8 patients, and in 3 these changes involved a loss of induration of 50% or more or actual disappearance of lesions. On the other hand, such single-dose therapy only resulted in a minimal and irregular decrease in the percentage of viable M. leprae in the dermis, and in no instance complete clearance of viable M. leprae by the methods utilized. Therefore, we suspect that the rapid clinical improvement, as we postulated earlier for the observed relative absence of ENL in the minocycline-treated patients, is not primarily a result of its antimicrobial activity but is more likely due to its known multiple effects on inflammation (16).
In these study patients the rate of loss of viable M. leprae from the skin at 1, 2 and 3 months after the initiation of therapy was remarkably similar to what we found previously with 100 mg daily (6), all patients being devoid of viable M. leprae by the same methods utilized in both studies only after 3 months' therapy. As in our previous trial with 100 mg daily, this was a faster rate of clearance of viable M. leprae than had been found with either dapsone or clofazimine therapy (14), but slower than for rifampin (14,15). We conclude that, because of the observed increased number of side effects found with larger single doses and twice daily therapy, there is little reason to advocate larger doses of minocycline than 100 mg once daily for lepromatous leprosy patients.
This study provided no evidence that a single, large, 200-mg dose of minocycline resulted in killing of M. leprae. Because in this trial the viability of M. leprae foot pad inocula of less than 5000 bacilli were not evaluated, modest killing of M. leprae could unfortunately have been missed. In M. lep rae-infected mice, although minocycline therapy 3 days weekly and once weekly was consistently active against several M. leprae strains, therapy 1 day monthly was not (7). Furthermore, although in mice single-dose minocycline could be demonstrated to be active against M. leprae, that activity was certainly no more than modest (18). Since in these leprosy patients we found that single-dose minocycline does not result in impressive killing of M. leprae, it docs not appear that it could be administered once monthly with rifampin if the goal is to shorten the duration of therapy necessary to effectively treat leprosy patients.
In this study we have found minocycline therapy to be once again impressively effective in clinical trial. Currently, it could be recommended to replace one of the generally utilized and limited number of antimicrobials available to treat leprosy patients, namely, dapsone, clofazimine, rifampin, and the thioamides. This might be of especial utility when there arc suspected problems, with these established agents, of drug resistance, drug allergy, or drug intolerance. Furthermore, because minocycline has been found consistently bactericidal for M. leprae in mice and patients, it has the potential to provide over previously established agents another companion bactericidal drug to rifampin, perhaps permitting shorter-course therapy, the key to that success in pulmonary tuberculosis being the use of two bactericidal agents (1). Although also in recent years, fluoroquinolones (9,10) and clarithromycin (4,11) have proved bactericidal for M. leprae in mice and in clinical trial, unlike minocycline where there is considerable experience with its safety for the long periods required of any treatment of leprosy, there is no such parallel experience with these agents. Thus, of the three newer classes of antibiotics recently found effective against M. leprae, minocycline appears currently the most acceptable for clinical application.
Acknowledgment. This investigation received financial support from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and from a grant from the Gillis W. Long Hansen's Disease Center, Carville, Louisiana, U.S.A.
REFERENCES
1. EAST AFRICAN/BRITISH MEDICAL RESEARCH COUNCIL STUDY. Results at 5 years of a controlled comparison of a 6-month and a standard 18-month regimen of chemotherapy for pulmonary tuberculosis. Am. Rev. Respir. Dis. 116(1977)3-8.
2. FAJARDO, JR., T., VILLAHERMOSA, L., DELA CRUZ, E. G., ABALOS, R., FRANZBLAU, S. and WALSH, G.P. Minocycline in lepromatous leprosy. Int. J. Lepr. (in press).
3. FANNING, W. L., GUMP, D. W. and SOFFERMAN, R. A. Side-effects of minocycline: a double blind study. Antimicrob. Agents Chemother. 11(1977)712-717.
4. FRANZBLAU, S. G. and HASTINGS, R. C. In vitro and in vivo activities of macrolides against Mycobacterium leprae. Antimicrob. Agents Chemother. 32(1988)1758-1762.
5. GELBER, R. H. Activity of minocycline in Mycobacterium leprae -infected mice. J. Infect. Dis. 156(1987)236-239.
6. GELBER, R. H., FUKUDA, K., BYRD, S., MURRAY, L. P., Siu, P., TSANG, M. and REA, T. H. A clinical trial of minocycline in lepromatous leprosy. Br. Med. 304(1992)91-92.
7. GELBER, R. H., SIU, P., TSANG, M., ALLEY, P. and MURRAY, L. P. Effect of low-level and intermittent minocycline therapy on the growth of Mycobacterium leprae in mice. Antimicrob. Agents Chemother. 35(1991)992-994.
8. GELBER, R. H., SIU, P., TSANG, M. and MURRAY, L. P. Minimal bactericidal dietary concentration of minocycline for Mycobacterium leprae- infected mice is very low and similar to its minimal inhibitory dietary concentration. Int. J. Lepr. 60(1992)276-277.
9. GROSSET, J. H., Ji, B., GUELPA-LAURAS, C.-C, PERANI, E. G. and N'DELI, L. N. Clinical trial of Pefloxacin and ofloxacin in the treatment of lepromatous leprosy. Int. J. Lepr. 58(1990)281-295.
10. GUELPA-LAURAS, C. C, PERANI, E. G., GIROIR, A. M. and GROSSET, J. H. Activity of Pefloxacin and ciprofloxacin against Mycobacterium leprae in the mouse. Int. J. Lepr. 55(1987)70-77.
11. JI, B., JAMET, P., PERANI, E. G., BOBIN, P. and GROSSET, J. H. Powerful bactericidal activities of clarithromycin and minocycline against Mycobacterium leprae in lepromatous leprosy. J. Infect. Dis. 168(1993)188-190.
12. JI, B., PERANI, E. G. and GROSSET, J. H. Effectiveness of clarithromycin and minocycline alone and in combination against experimental Mycobacterium leprae infection in mice. Antimicrob. Agents Chemother. 35(1991)579-581.
13. JI, B., PERANI, E. G., PETINON, C. and GROSSET, J. H. Bactericidal activities of single or multiple doses of various combinations of new antileprosy drugs and/or rifampin against M. leprae in mice. Int. J. Lepr. 60(1992)556-561.
14. SHEPARD, C. C. A brief review of experiences with short term clinical trials monitored by mouse foot pad inoculation. Lepr. Rev. 52(1981)299-308.
15. SHEPARD, C. C , LEVY, L. and FASAL, P. Rapid bactericidal effect of rifampicin on M. leprae. Am. J. Trop. Med. Hyg. 21(1972)446-449.
16. SUOMALAINEN, K., SORSA, T., GOLUB, L. M., RAMAMURTHY, N., LEE, H-M., UITTO, V-J., SAARI, H. and KONTTINEN, Y. T. Specificity of the anticollaginase action of tetracyclines; relevance to their anti-inflammatory potential. Antimicrob. Agents Chemother. 36(1992)227-229.
17. WHO EXPERT COMMITTEE ON LEPROSY. Sixth report. Geneva: World Health Organization, 1988. Tech. Rep. Ser. 768.
18. XIONG, J., JI, B., PERANI, E. G., PETINON, C. and GROSSET, J.-H. Further study of the effectiveness of single doses of clarithromycin and minocycline against Mycobacterium leprae in mice. Int. J. Lepr. 62(1994)37-42.
1. M.D.; San Francisco Regional Hansen's Disease Program, San Francisco, CA 94115, U.S.A.
2. San Francisco Regional Hansen's Disease Program, San Francisco, CA 94115, U.S.A.
3. San Francisco Regional Hansen's Disease Program, San Francisco, CA 94115, U.S.A.
4. San Francisco Regional Hansen's Disease Program, San Francisco, CA 94115, U.S.A.
5. M.D., Department of Dermatology, The University of Southern California School of Medicine, Los Angeles, CA 90033, U.S.A.
Reprint requests to R. H. Gelber, M.D., 2211 Post St., Suite 301, San Francisco, CA 94115, U.S.A.
Received for publication on 14 June 1994;
Accepted for publication in revised form on 10 August 1994.