- Volume 62 , Number 4
- Page: 574–9
Lipid composition of the stratum corneum of the sole in patients with leprosy
ABSTRACT
Several reports support the view that changes of composition of the stratum corneum (SC) lipids may be the cause of impaired barrier function which, in turn, gives rise to xerosis and ichthyotic skin in leprosy. Many reports about abnormalities of serum lipids and cutaneous manifestations, such as xerosis and ichthyotic changes in leprosy, led us to the idea that the composition of SC lipids in patients with leprosy may be different f rom that in normal subjects. However, the many studies done in the past do not sufficiently account for this. To investigate the composition of SC lipids in patients with leprosy, thin-layer chromatography (TLC) was undertaken. Extraction of the SC lipids with a methanolchloroform-H20 mixture (4:2:1.6, v/v/v, Bligh-Dyer solvent) was carried out after shaving of the SC f rom the sole. TLC was performed and the composition of lipids was quantitated by photodensitometry. Our study revealed that the composition of SC lipids in the anesthetic lesions of leprosy patients was higher in cholesterol sulfate and triglycerides and lower in sphingolipids and cholesterol esters than that of normal subjects.RÉSUMÉ
Différents rapports supportent l'idée que des modifications dans la composition du stratum corneum (SC) en lipides peuvent être la cause de la détérioration de la fonction de barrière, ce qui, à son tour, donne lieu au xerosis et à la peau ichthyotique dans la lèpre. De nombreux rapports sur les anomalies des lipides sériques et des manifestations cutanées telles que le xerosis et des modifications ichthyotiques dans la lèpre ont conduit à l'idée que la composition en lipides du SC chez les patients lépreux pourrait être différente de celle des sujets en bonne santé. Cependant, les nombreuses études réalisées dans le passé ne sont pas sufisamment probantes.On a utilisé la chromatographic en couche mince (CCM) pur analyser la composition lipidique de SC des malades de la lèpre. L'extraction des lipides du SC a été réalisée avec un mélange de methanol-chloroforme-H.O (4:2:1.6, solvant de Bligh-Dyer après décapage du SC plantaire. La CCM a été réalisée et la composition en lipides a été quantifiée par photodensitométrie. Notre étude a révélé que la composition du SC en lipides au niveau des lésions anesthétiques des malades de la lèpre était plus riche en sulfate de cholesterol et en triglycérides, et moins riche en sphingolipides et en esters de cholesterol que celle de sujets normaux.
RESUMEN
Hay vários reportes que apoyan la idea de que los câmbios en la composición lipídica dei estrato córneo pueden ser causa de disfunción meccánica de la piei, y cl orígen de la xerosis y la ictiosis de la lepra. Esto nos condujo a suponer que la composición lipídica del estrato córneo en los pacientes con lepra podría ser diferente de aquella en los sujetos sanos. En este estúdio se utilizo la cromatografia en capa fina (TLC) para analizar la composición lipidica del estrato córneo de la piei de la planta dei pie de paccintes con lepra. La extracción de lípidos se hizo utilizando una mezcla de metanol-cloroformo-agua (4:2:1.6, solvente de Bligh-Dyer). Los lípidos extraídos se analizaron por TLC y se cuantificaron por fotodensítometría. El estúdio revelo que la composición lipídica dei estrato córneo de las lesiones anestésicas de los pacientes con lepra, fue más rica en sulfato de colesterol y en triglicéridos y más pobre en esfingolípidos y en ésteres del colesterol que la composición lipídica de los sujetos sanos.The stratum corneum (SC), the uppermost layer of the skin which is in direct contact with the external environment, consists of protein-rich corneocytes and SC lipids dispersed between corneocytes (7). Present studies have revealed the important roles of the SC in protecting the human body from water loss and external injuries. Human skin surface lipids consist of sebum originating from the sebaceous glands (23,30) and epidermal lipids from the keratinizing epidermis (1,5). Many reports have noted the importance of SC lipids not only for their barrier function or water-holding capacity (8,19,29) but for their role in the cohésion-desquamation of keratinocytes (6,20). The sole of the foot, though it normally carries some sebum transferred from other areas, is devoid of sebaceous glands so that epidermal lipids free of sebum can be obtained by separating the SC from the skin of the sole.
Several lines of evidence suggest that the composition and amount of SC lipids in patients with leprosy are different from those of normal healthy individuals (3,10,15,16,22). Many studies have documented the abnormalities of blood lipids in leprosy patients in considerable detail, and clinical manifestations of xerotic skin and ichthyosiform changes which imply abnormalities of SC lipids in them have been reported (25). However, no precise report about the composition of SC lipids in patients with leprosy has ever been made.
To evaluate the biochemical alteration of SC lipids in patients with leprosy, we herein compare the difference between the SC lipids of anesthetic or nonanesthetic soles in patients with leprosy and those of the soles in normal healthy individuals.
MATERIALS AND METHODS
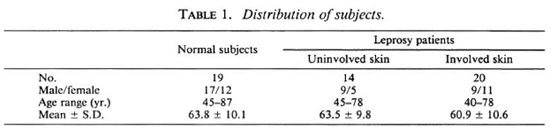
Materials The SC was taken from the soles of patients with lepromatous or borderline lepromatous leprosy who were inactive clinically and negative bacteriologically. The patients had received multidrug therapy (MDT) including clofazimine. However, they had not received any antileprosy medication during the last year. The sole regions included: 1) involved skin, i.e., hypoesthetic or anesthetic soles of 20 leprosy patients (40-78 years of age, mean 60.9 ±10.6 years), and 2) uninvolved sole skin with normal sensation of 14 patients (47-78 years of age, mean 63.5 ±9.8 years). As a control group, similar samples were obtained from 19 age-matched, healthy persons for comparison (Table 1). We shaved off the superficial SC from the sole and took a midportion of the SC as a sample in thin slices (1mm thick) with a surgical scalpel.
Those with systemic diseases, such as diabetes mellitus, hypertension and hyperlipidemia which may cause abnormalities of the skin or blood lipids, were excluded in the selection of subjects. The subjects were left untreated topically for at least 2 weeks before sampling of the SC.
Methods
Extraction of lipids. To extract the SC lipids, sampled SCs from the sole were minced into smaller pieces about as small as 1 mm3 and soaked overnight in 5 ml of BlighDyer solution (chloroform-methanol-H20 = 4:2:1.6 v/v/v) (2). After vortexing several times the supernatant was gathered into a tube. The SCs were immersed again in 7 ml of chloroform-methanol mixture (2:1 v/v) and homogenized in a glass homogenizer. The supernatant and homogenized SC were filtered together through Whatman No. 3 filter paper yielding a 3-4-ml filtrate. After the addition of 2 ml of distilled water, the filtrate was centrifuged at 2000 x g for 10 min. The upper aqueous phase was removed carefully with a Pasteur pipette and the lower phase, which contained the lipids mixture, was then evaporated to dryness on a 35ºC warm plate in the air. The lipid extract, SC lipids mixture, was stored at - 20ºC until used.
Standards and TLC plate. Two µ g/ µ l of cholesterol, cholesteryl ester (18:1), oleic acid (18:1), oleic acid methyl ester (18:1) and triolein (18:1) each were used for lipid standards for chromatography, and 1 µ g/ µ l each of galactocerebrosides type I and II and ceramides type III and IV were used for sphingolipid standards. Cholesterol 3-sulfate was diluted to 2.5 µ g/ µ l for a cholesterol sulfate standard (all these lipid standards were purchased from Sigma Chemical Co., St. Louis, Missouri, U.S.A.). For thin-layer chromatography (TLC), TLC precoated plates® (20 x 20 cm, mean pore 60Å in diameter) coated with silica gel (Sigma) were used for the separation of the lipid mixtures.
Spotting of lipid sample. Before use, the TLC plates were prewashed in a vertical developing chamber (American Optical, Buffalo, New York, U.S.A.) by the first developing solvent mixture (chloroformmethanol-H20 = 95:20:1 v/v/v) to eliminate contaminants on the plates, and then activated at 110ºC for more than 2 hr. One hundred µ g of the lipid samples dissolved in 40 µ g of chloroform-methanol mixture (2:1 v/v) were spotted onto the bottom edge of the plate at a constant distance of 2 cm, using Hamilton syringes. Ten µ l each of the above three lipid standards were applied respectively on the peripheral lanes of each plate.
Chromatography. For fractionation of polar lipids, a spotted TLC plate was developed with the first solvent mixture (chloroform-methanol-H2O = 95:20:1 v/v/v) to a distance of 5.5 cm from the origin spotted. After the first development, the chromatographed plate was dried thoroughly in cool air. The second solvent system, hexane-ethyl ether-acetic acid mixture (80:20:10 v/v/v), was developed to 9 cm for fractionation of the neutral lipids. This plate was dried by warm air for a longer period (about 20 min), ensuring near-complete evaporation of the acetic acid. The third solvent, petroleum ether, was developed to 15 cm for the nonpolar lipids and dried by warm air. All chromatography manipulations were done at room temperature (22ºC).
Charring. After complete drying, the chromatographed plate was sprayed with 10% CuSO4 in an 8% H3PO4 aqueous solution and charred at 170ºC in the oven (Isotemp Model 281; Fisher Scientific Co., Pittsburgh, Pennsylvania, U.S.A.) for about 25-30 min until appropriate charring was achieved.
Analysis. The charred lipid fractions were quantitated by a photodensitometer (Cliniscan-2; Helena Labs, Beaumont, Texas, U.S.A.) in an absorbance mode of 570 nm wavelength, and the mean and standard deviations were calculated by weight percent. The Student's t test was used to calculate the statistical significant differences observed. One lane of chromatography and its corresponding result by photodensitometry is shown in The Figure.
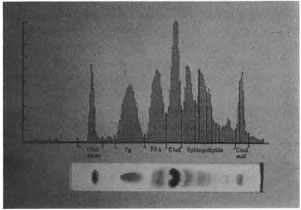
The figure. One lane of chromatography and the corresponding result of photodensitometry.
RESULTS
Comparisons of each fraction of the SC lipids from the sole between involved or uninvolved skin of leprosy patients and those of healthy subjects are shown in Tables 2 and 3. The sphingolipid content of the SC was significantly reduced in both involved (p < 0.01 ) and uninvolved (p < 0.01 ) skin in patients with leprosy compared with that of healthy subjects.
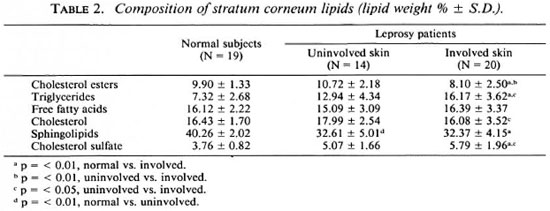
Cholesterol sulfate content of the skin (involved and uninvolved) of the leprosy patients was higher than that of healthy subjects (p < 0.01).
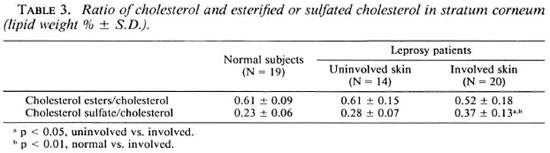
A significant increase of the ratio of cholesterol sulfate to cholesterol was observed in the involved skin in comparison with uninvolved skin (p < 0.05) and with healthy subjects (p < 0.01).
DISCUSSION
It is well known that skin-surface lipids are of complex origin, and most of them come from the sebum and SC of the epidermis (1,5). SC could be explained simplistically by a "brick and mortar" model which equates the lipid-depleted corneocytes with bricks and the lipid-repleted intercellular substances with mortar (6,9). Recent cytochemical and freeze-fracture studies indicate that epidermal intercellular lipids derived from the secreted contents of epidermal lamellar bodies are implicated in the permeability barrier in the skin and cohesion-desquamation of the keratinocytes.
Contrary to the lipids from sebaceous origin (7,23) no remarkable variability was found in the amount and composition of SC lipids, depending on the body site sampled. But biochemical changes of lipids, such as saturation and chain elongation of fatty acids (30), acylation of sphingolipids, and esterification and sulfation of free sterol, were remarkable as the epidermal cells move from the basal layer to the horny layer (9,11,18). The SC lipids constitute 6%-12% of total SC by weight (7) and are mainly composed of ceramides, cholesterol and its esters, and free fatty acids (4,5,28). Abnormalities of the composition of SC lipids have been reported in patients with ichthyosis, psoriasis and atopic dermatitis.
Sphingolipids (mainly ceramides, glucosylceramides and sphingomyelin) increase in amount during keratinization and increase progressively as the SC gets thicker (11,17,18). Recently, it has been reported that sphingolipid plays a most important role in the regulation (28) of the barrier function of the epidermis and mediates stability of the lipid bilayer in cooperation with cholesterol sulfate. Sphingolipid content (especially ceramides) significantly declined in patients with atopic dermatitis (13) and senile xerosis (24), who show xerotic or ichthyosiform skin so that there is an increase of transepidermal water loss (TEWL) and structural abnormalities of the lamellar body, such as an increase of the relative volume of lamellar granules and their vesicular form lacking lamellated internal structure (7). We suspect that leprosy, which shows xerosis and ichthyosiform changes, also may exhibit similar changes. In our results the ratio of sphingolipid to total lipid in both involved and uninvolved skin of leprosy patients was significantly lower than that of the normal population.
Because cholesterol sulfate is degraded into cholesterol by cholesterol sulfatase during cornification (17), the level of cholesterol sulfate was significantly reduced in the desquamated material (20). The results are in accord with the assertion that cholesterol sulfate, which is the most polar lipid in the SC, serves in the coupling and stabilizing of the lipid bilayer which, in turn, sustains cellto-cell cohesion within the SC, and its hydrolysis may be necessary to permit shedding of cells from the skin surface. So it is proposed that cholesterol sulfate might play an important part in the cohesion-desquamation reaction of corneocytes by its role in intercellular cement substance. This assumption is based on the abnormal degradation of cholesterol sulfate resulting from a genetic lack of steroid sulfatase in recessive X-linked ichthyosis which shows delayed desquamation and an abnormal barrier function to water diffusion. Also, the fact that daily application of cholesterol suitably incorporated into stable preparations leads to a total regression of the clinical symptoms of ichthyosis explains the above assumption (1). Our data indicate increased cholesterol sulfate in leprosy patients, especially in involved skin. Based upon these results, we speculate that the accumulation of cholesterol sulfate in the SC may be implicated in the ichthyotic changes and hyperkeratosis as shown in recessive X-linked ichthyosis.
The ratio of cholesterol sulfate to cholesterol may be the primary lipid couple involved in the corneocyte-to-corneocyte attachment at the time when desmosome structures disappear or become nonfunctional (1). Thus, a significant increase of this ratio means an abnormal desquamation of the SC. Our results also indicate that this ratio is more increased in the involved skin of leprosy patients than in their uninvolved skin and than in normal subjects.
Evidence has been accumulating for a long time that there might be various changes in the composition of SC lipids as well as in other organs in patients with leprosy. This was supported by the following findings: First, skin manifestations such as xerosis, hyperkeratosis and ichthyosiform changes of the skin in patients with leprosy, particularly in involved skin, showed the conditions of deranged cornification (25). Second, numerous reports noted abnormalities of the lipid fractions of blood and several organs in leprosy patients (3,10,15,16,22). The SC lipid changes in the involved skin of leprosy may be explained by the fact (20) that a disturbance of cornification and a diminution of sweating, caused by nerve involvement in leprosy, may induce abnormal changes. Furthermore, electron-microscopic studies have described various changes in the intraneural blood vessels, such as disruption in endothelial continuity, thickening and reduplication of the basement membrane of capillaries, and edema of the vessel walls resulting in occlusion of their lumina, potentially causing depletion of raw material for lipid biosynthesis.
Several reports about the characteristics of blood lipids in leprosy patients have been published (3,10,16,22). However, no consensus has been reached about the lipid composition of the blood and various organs in patients with leprosy. Moreover, serum lipids do not directly reflect the SC lipids because the bulk of epidermal lipids is derived from the skin itself, and skin-produced lipids do not participate in the general circulation. Hence, the relationship between the lipid composition of the blood and SC in leprosy patients remains unclear. By lipid analysis, we could observe lipid composition changes, such as sphingolipids, cholesterol sulfate, in involved or uninvolved skin of leprosy patients. A more trivial explanation for the findings would be that the anesthetic sole undergoes more trauma than a sole with normal sensation due to the simple lack of pain sensation. This could cause callus formation, and would cause biochemical changes. However, we can see the changes of lipid composition in the nonlesional soles as well as lesional soles. The reasons for these changes are unknown exactly and, in addition to peripheral nerve palsy and disturbance of blood circulation, generalized metabolic disturbance of the lipids may participate in these changes.
REFERENCES
1. BROD, J. Characterization and physiologie role of epidermal lipids. Int. J. Dermatol. 30(1991)84-90.
2. BLIGH, E.G. and DYER, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(1959)911-917.
3. CHEKHERDEMIAN, M. Determination of cholesterol, phospholipids, total lipids and total protein and glycoprotein and lipoprotein by electrophoresis in the distinct form of leprosy. Leprologica 5(1960)69-70.
4. DOWNING, D.T. In vivo studies of cutaneous lipid biosynthesis. Semin. Dermatol. 11(1992)162-168.
5. DOWNING, D.T., STEWART, M.E., WERTZ, P.W., COLTON, S.W. and STRAUSS, J.S. Skin lipids. (Minireview) Comp. Biochem. Physiol. 76B(1983)673-678.
6. ELIAS, P.M. Epidermal lipids, barrier function, and desquamation. J. Invest. Dermatol. 80(1983)44s-49s.
7. ELIAS, P.M. Plastic wrap revisited: the stratum corneum two-compartment model and its clinical implication. Arch. Dermatol. 123(1987)1405-1406.
8. ELIAS, P.M., COOPER, E.R., KORC, A. and BROWN, B.E. Percutaneous transport in relation to stratum corneum structure and lipid composition. J. Invest. Dermatol. 76(1981)297-301.
9. FREINKEL, R.K. Lipids of epidermis. In: Fitzpatrick, T.B., Eisen, A.Z., Wolff, K., Freedberg, I.M. and Austen, K.F. Dermatology in General Medicine. 3rd edn. New York: McGraw-Hill International Book Co., 1987, pp. 191-194.
10. GOKHALE, S.K. and GODBOLE, S.H. Serum lipolytic enzyme activity and scrum lipid partition in leprosy and tuberculosis. Indian J. Med. Res. 45(1957)327-336.
11. GRAY, G.M. and YARDLEY, H.J. Different populations of pig epidermis; isolation and lipid composition. J. Lipid Res. 16(1975)441-447.
12. GRUBAUER, G., FEINGOLD, K.R., HARRIS, R.M. and ELIAS, P.M. Lipid content and lipids type as determinants of the epidermal permeability barrier. J. Lipid Res. 30(1989)89-96.
13. IMOKAWWA, G., ABE, A., JIN, K., HIGAKI, Y., KA-WASHIMA, M. and HIDANO, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin? J. Invest. Dermatol. 96(1991)523-525.
14. JOB, C.K. Nerve damage in leprosy. (Editorial) Int. J. Lepr. 57(1988)532-539.
15. KIM, N.H., SUH, S.B. and CHUNG, T.H. Determination of serum cholesterol, phospholipids and triglycerides in leprosy patients. Kyungpook Univ. Med. J. 20(1979)468-473.
16. KUSAKA, T. Alteration in the lipid content of the blood and tissues in leprosy patients. La Lepro 27(1958)228-232.
17. LAMPE, M.A., BURLINGAME, A.L., WHITNEY, J., WILLIAMS, M.L., BROWN, B.H., HOITMAN, and ELIAS, P.M. Human stratum corneum lipids; characterization and regional variations. J. Lipid Res. 24(1983)120-130.
18. LAMPE, M.A., WILLIAMS, M.L. and ELIAS, P.M. Human epidermal lipids; characterization and modulations during differentiation. J. Lipid Res. 24(1983)131-140.
19. LANDMANN, L. Epidermal permeability barrier; Transformation of lamellar granule-disks into intercellular sheets by a membrane-fusion process, a freeze-fracture study. J. Invest. Dermatol. 87(1986)202-209.
20. LONG, S.A., WERTZ, P.W., STRAUSS, J.S. and DOWNING, D.T. Human stratum corneum polar lipids and desquamation. Arch. Dermatol. Res. 277(1985)284-287.
21. MENON, G.K., GRAYSON, S. and ELIAS, P.M. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J. Invest. Dermatol. 86(1986)591-597.
22. MISRA, U.K . and VENKITASUBRAMANIAN, T.A. Scrum lipids in leprosy by silicic acid column chromatography. Int. J. Lepr. 32(1964)248-259.
23. NICOLLAIDES, N. Skin lipids; their biochemical uniqueness. Science 186(1974)19-26.
24. SAINT-LEGAR, D., FRANCOIS, A.M., LEVEQUE, J.L., STOUDEMAYER, T.J., KLIGMAN, A.M. and GROVE, G. Stratum corneum lipids in skin xerosis. Dermatologica 178(1989)151-155.
25. SCHULZ, E.J. Ichthyosiform conditions occurring in leprosy. Br. J. Dermatol. 77(1965)151-157.
26. TEZUKA, T. Electron-microscopic changes in xerosis senilis epidermis; its abnormal membranecoating granule formation. Dermatologica 166(1983)57-61.
27. WERNER, Y., LINDBERG, M. and FORSLIND, B. Membrane-coating granules in 'dry' non-eczematous skin of patients with atopic dermatitis. Acta. Dermatol. Venereol. (Stockh.) 67( 1987)385-390.
28. WERTZ, P.W. Epidermal lipids. Semin. Dermatol. 11(1992)106-113.
29. WERTZ, P.W., SWARTZENDRUBER, D.C., ABRAHAM, W., MADISON, K.C. and DOWNING, D.T. Essential fatty acids and epidermal integrity. Arch. Dermatol. 123(1987)1381-1384.
30. ZIBOH, V.A. and CHAPKIN, H.S. Metabolism and function of skin lipids. Prog. Lipid Res. 27(1988)81-105.
1. M.D.; Departments of Dermatology and Immunology, Kyungpook National University School of Medicine, 52 Samduk 2-Ka, Choong-Ku, Taegu City, South Korea 700-412.
2. M.D.; Departments of Dermatology and Immunology, Kyungpook National University School of Medicine, 52 Samduk 2-Ka, Choong-Ku, Taegu City, South Korea 700-412.
3. M.D.; Departments of Dermatology and Immunology, Kyungpook National University School of Medicine, 52 Samduk 2-Ka, Choong-Ku, Taegu City, South Korea 700-412.
4. M.D.; Departments of Dermatology and Immunology, Kyungpook National University School of Medicine, 52 Samduk 2-Ka, Choong-Ku, Taegu City, South Korea 700-412.
5. M.D., Departments of Dermatology and Immunology, Kyungpook National University School of Medicine, 52 Samduk 2-Ka, Choong-Ku, Taegu City, South Korea 700-412.
Reprint requests to Dr. Do Won Kim, Department of Dermatology.
Received for publication on 15 December 1993;
Accepted for publication in revised form on 28 July 1994.